Cell culture expansion for biotherapeutic development has traditionally involved a series of time-consuming, labor-intensive batch cultures that are susceptible to contamination. Cell culture expansion using a perfusion process promises easy scalability, ensures high productivity gains, and saves time and costs during large-scale biomanufacturing.
Cell culture is a crucial tool in the biotech sector. Applications range from in vitro studies in basic research and generating cell banks for clinical research, to producing biologic therapies for use in humans.
Small laboratories and global biomanufacturing companies alike, are continuously optimizing cell culture expansion methods to efficiently produce large quantities of viable cells in the safest, most cost-effective way possible.
The trick to keeping cells healthy and viable is to provide an optimal physical environment. To produce cells that grow as expected, the right temperature, pH, physiological molarity, oxygen supply, and carbon dioxide pressure need to be maintained. Moreover, shear stress – a force that causes cells to rupture – has to be prevented.
In the biomanufacturing setting the same requirements apply, despite the shift to a larger scale. Cell culture expansion in manufacturing typically involves an ‘expansion seed train’. This seed train uses a sequence of progressively larger cell cultivation systems that help increase viable cell numbers to generate the target number of cells.
Challenges in traditional cell culture expansion
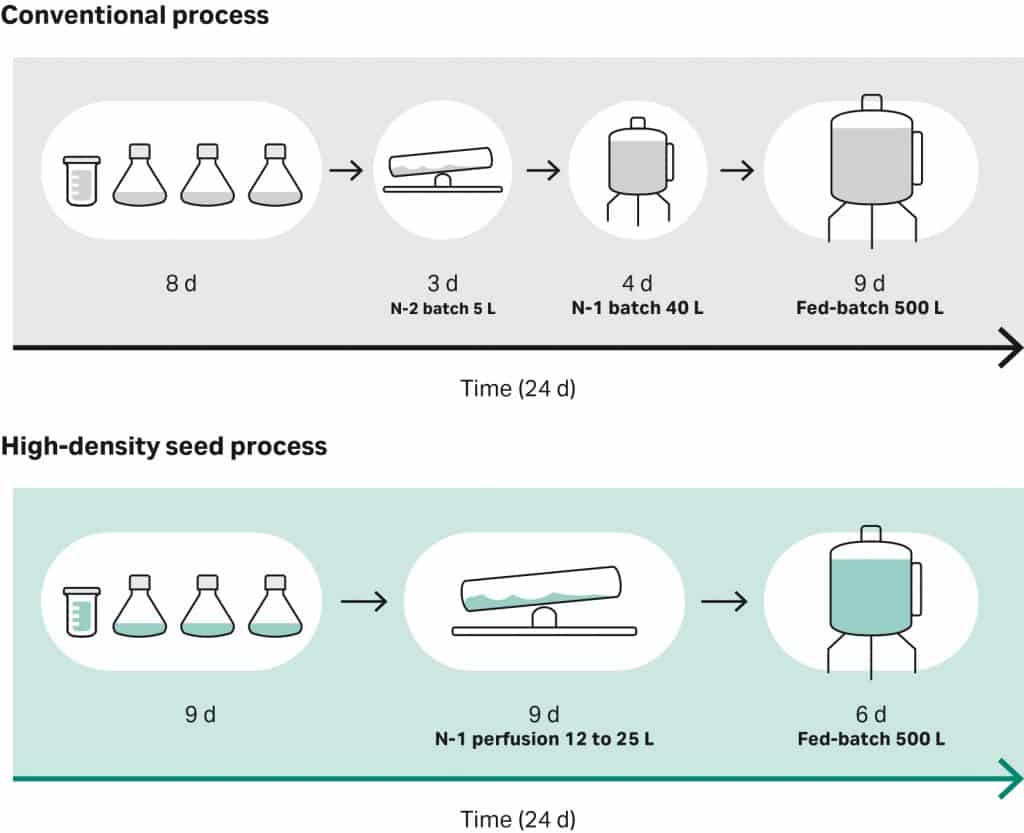
Conventionally, the entire cell expansion process takes about three to four weeks and starts with a vial of viable cells transferred into a series of shake flasks. The cells are moved from the flask into a rocking bioreactor and then into a stirred tank. Next, the cells are transferred to a final seed bioreactor, also known as the N-1 bioreactor. Finally, the cells are transferred into the production bioreactor, which produces the desired cell volumes.
The final stage in the production bioreactor spans from 10 to 14 days. The majority of the cell culture expansion process and product formation happens in this final production bioreactor. Therefore, this stage restricts the speed of the entire cell expansion process and creates a bottleneck in terms of output in a production plant.
Apart from being labor- and time-intensive, another limitation of this traditional cell culture expansion process are the manually operated transfer stages, which heighten the risk of contamination.
Perfusion N-1: the key to successful cell culture expansion
Scientists have suggested two ways of overcoming these challenges. First, by reducing the number of transfer stages within the expansion seed train, and second, by introducing higher density cell cultures into the production bioreactor.
One method to single-handedly accomplish both of these improvements is by running the N-1 bioreactor in perfusion mode, which is known as the N-1 perfusion process.
During N-1 perfusion, cells are retained and cultured in a specialized single-use bioreactor bag within the penultimate bioreactor. The initial stages are replaced with one bioreactor continuously supplied with fresh cell culture media, which is optimized for high cell viability and growth.

“Perfusing the cells in the bioreactor mimics the concept of perfusing an organ in the body, by supplying fresh nutrients on one end, and removing metabolic waste products on the other end,” said Andreas Castan, Strategic Technology and Business Development Leader at Cytiva.
Running perfusion mode enables higher cell densities to be achieved earlier on in the expansion process, prior to the introduction of the culture into the production bioreactor. This, in turn, allows culture times to be shifted, reducing the overall run time in the production bioreactor.
Apart from reducing dependency on the production bioreactor by producing more cells earlier on in the expansion process, perfusion also has other advantages. It produces a comparable harvest (if not higher) to the conventional cell culture process, while shortening the fed-batch step by three to five days.
By eliminating the number of transfers and implementing single-use workflows, the perfusion process uses less consumables and reduces the number of manual operations, indirectly reducing the risk of contamination as well.
The biggest hurdle to running N-1 bioreactor perfusion in the past was the complicated setup. However, modern cell production systems are now capable of incorporating perfusion into the bioreactor by altering the mode of operation and changing the type of bioreactor bag responsible for cell culture expansion.
Choosing the right platform to enable perfect perfusion
The key to successful perfusion is working with platforms that provide the right range of physical parameters, which maintain cells at a high viability and in exponential growth, explained Castan. The systems that are ideal to use are those designed with the user and the perfusion process in mind from the beginning.
For example, Cytiva’s ReadyToProcess WAVE 25 bioreactor system has an integrated perfusion filter. It also has perfusion methods predefined in the system’s control software, making it easy to use.
“If larger volumes are desired, customers can now transfer their process from the WAVE system to our recently launched Xcellerex Automated Perfusion System (APS). The low-shear design and the use of automation in both systems are the key enablers of the intensification effort,” Castan elaborated.

Neil Ross, Senior Global Marketing Manager at Cytiva, explained that prior to the release of Cytiva’s Automated Perfusion System, users were tasked with putting their own perfusion solution together.
With the Xcellerex APS system, all operations required to run a perfusion process are fully integrated, automated, and monitored in real-time.
Along with ease of use, the systems can also be instrumental in aiding process development, added Ross: “Our users can develop their process for N-1 using the WAVE and with confidence know that if they need to inoculate this at 10,000-liter bioreactor volumes, they can scale that process up in the Xcellerex APS system.”
In fact, scalability is a significant benefit of the perfusion approach compared to conventional cell culture expansion methods. While the WAVE system is ideal to use for pilot-scale drug development projects, the Xcellerex APS system can feed into commercial production bioreactors.
Enabling a future of continuous large-scale biomanufacturing
Today, seed train perfusion is increasingly being applied for intensifying seed trains as well as cell banking, explained Castan. But perfusion can also be used for consistently producing proteins, monoclonal antibodies, or other active biopharmaceutical ingredients.
This is achieved via a process known as ‘steady-state perfusion’, which allows perfusion to continue for longer periods from anywhere between 30 to 100 days.
Optimizing the steady-state perfusion process has generated a lot of interest lately. This is because steady-state perfusion could help facilitate continuous manufacturing, a method that enables the constant production of products to maximize the productivity of a facility.
According to Castan, hybrid processes that involve both perfusion and conventional cell culture expansion are also gaining momentum.
“Hybrid processes are a way of achieving continuous manufacturing with significant productivity gains per unit volume, albeit with less investment from a complexity perspective,” concluded Ross.
“Many customers are interested in this and we will be looking at ways to further simplify perfusion and make it all the more accessible in the coming months and years.”
To learn more about innovative, cost-effective ways to reduce the complexity of your cell culture expansion process, visit the Cytiva website.
To understand how the recently launched Xcellerex APS system can be used to intensify cell culture processes in N-1, visit this page.
Header image via Shutterstock.com, all other images via Cytiva.
This article was originally published in June 2021.





