Newsletter Signup - Under Article / In Page
"*" indicates required fields
Allergan has signed a deal with Editas Medicine to access its CRISPR technology and license up to five of its programs in ocular disease.
The Irish Allergan has a certain tendency to go on shopping sprees. Its latest deal is a research and development alliance with the American Editas Medicine, which is on the side of Feng Zhang and the Broad Institute in a fierce patent battle over the IP of CRISPR technology.
Allergan will pay Editas €85M ($90M) upfront in exchange for exclusive access to the biotech’s ocular programs as well as the option to license up to five of them. For its part, Editas keeps the option to co-develop and co-promote up to two of those products in the US.
Editas Medicine has already attracted plenty of investors, raising a massive €89M in its IPO last year. The deal includes its lead program for Leber Congenital Amaurosis 10 (LCA10), a rare genetic disease that causes retinal degeneration and leads to blindness during childhood. The program is currently in pre-clinical development.
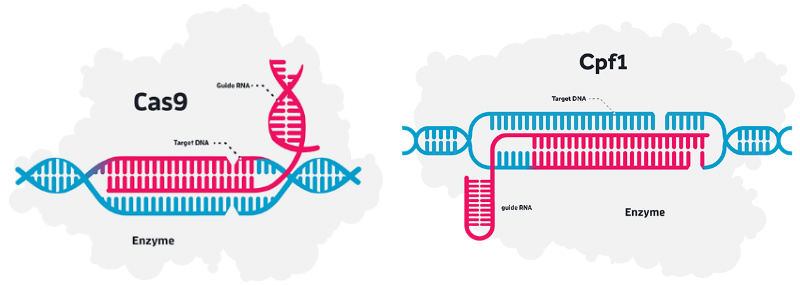
CRISPR is indeed creating plenty of hype in the biotech field as a tool that can be used to cut and replace virtually any gene with much less effort than any of its predecessors, TALEN and zinc finger proteins – though not always as precisely. With this new deal, Allergan will access both CRISPR/Cas9 and CRISPR/Cpf1 versions of the technology.
The great potential of the technology has spurred a fierce battle between two parties over the ownership of the technology’s IP. Recently, the US Patent Office ruled in favor of Feng Zhang and the Broad Institute, the owners of Editas Medicine’s licensed CRISPR patent. Their rival, Jennifer Doudna, who originally founded Editas with Zhang, cut ties with the company years ago due to their disagreement.
Despite huge expectations for the technology, there are still no results for CRISPR in humans and the first clinical trial was started in China only last November. Allergan is joining a group of investors that some would argue are purely gambling.
Images from vectorfusionart /Shutterstock; Editas Medicine
Are you interested in eye disease R&D?







