While studying a mutation connected to inflammatory disease, researchers from the University of Cambridge have found a previously unknown mechanism in immune response.
 As the cost of NGS plummets, genetic data is growing massively. With the help of bioinformatics, information about genetic variants and mutations can be linked to diseases. Sophia Genetics (Switzerland), Diploid (Belgium) and Saphetor (Switzerland) are examples of companies doing just that. This allows more precise diagnostics and prognosis.
As the cost of NGS plummets, genetic data is growing massively. With the help of bioinformatics, information about genetic variants and mutations can be linked to diseases. Sophia Genetics (Switzerland), Diploid (Belgium) and Saphetor (Switzerland) are examples of companies doing just that. This allows more precise diagnostics and prognosis.
However, exactly how specific variants contribute do disease is often not known. And this was what research led by the University of Cambridge tried to find out for a particular gene, C13orf31. Single-nucleotide mutations in this gene were associated with a larger risk of contracting leprosy – but also with higher incidence, some chronic inflammatory diseases.
In a study now published in Nature Immunology, the relation between the C13orf31 mutations and its impact on health is now shown. It involves a previously unknown mechanism in the regulation of macrophages (a type of immune cell), which has a significant impact on immune response.
In mice, the researchers created different C13orf31 mutations using CRISPR – the champion of gene editing tools and deemed ‘the scientific discovery of the century’. They showed that even tiny changes in the gene’s sequence made the mice more susceptible to blood infections (sepsis).
The deficiency in the immune system traces back to the role of the protein coded by C13orf31, which was unknown. It turns out it’s quite important: the protein acts as a central regulator of macrophages’ metabolism.
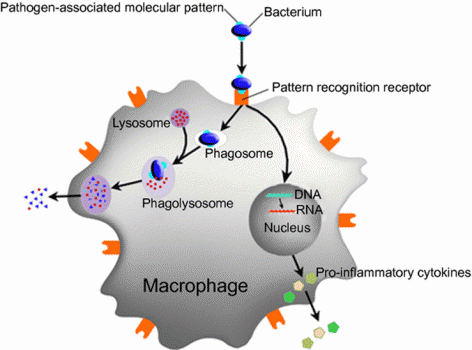
The protein was named FAMIN (short for Fatty Acid Metabolic Immune Nexus). By regulating how much energy is available to macrophages, a malfunction can impact the cells’ ability to kill bacteria. This results in the observed increased risk of leprosy and sepsis.
Additionally, macrophages’ regular functions include releasing cytokines, molecules that modulate inflammation. So, besides infectious disease, the mechanism is also relevant to autoimmune diseases.
Although there isn’t yet a strategy of how this finding could yield new therapies, this study is an example of how the flood of genetic data can guide breakthroughs in the understanding of human biology.
Feature Image Credit: Furnace (CC 2.0 Steven Lilley/Flickr)
Figure 1 Credit: Siezen and Hijum (2010) Genome (re‐)annotation and open‐source annotation pipelines. Microbial Biotechnology (doi: 10.1111/j.1751-7915.2010.00191.x)
Figure 2 Credit: Tran and Ramarao (2013) Bacillus cereus immune escape: a journey within macrophages. FEMS Microbiology Letters (doi: 10.1111/1574-6968.12209)





