Newsletter Signup - Under Article / In Page
"*" indicates required fields
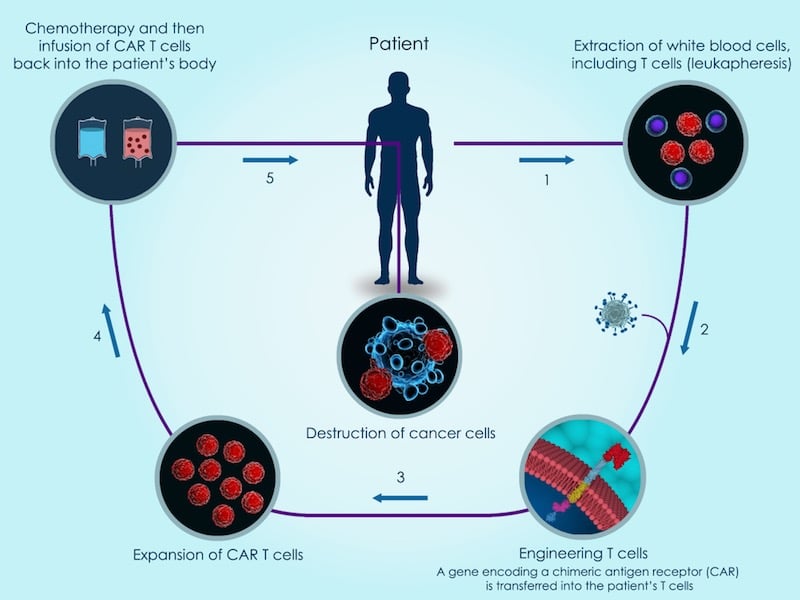
Celyad has reported clinical results showing the first time a patient has fully recovered from acute myeloid leukemia thanks to a CAR-T therapy, and without the need for chemotherapy preconditioning.
At the AACR meeting in Chicago this week, Celyad has presented preliminary data from three of its ongoing Phase 1 clinical trials with a new version of the popular CAR-T technology. The company makes T cells express a natural killer receptor that targets eight different tumor ligands simultaneously.
So far, Celyad’s CAR-T therapy, called CYAD-01, has shown a “favorable safety profile,“ without the life-threatening side effects, such as cytokine release syndrome and neurotoxicity, typical of most other CAR-T cells. Including Kymriah and Yescarta, the only two CAR-T therapies already on the market.
But, most importantly, Celyad has been the first to report that a patient with acute myeloid leukemia (AML) has been free of cancer so far for 9 months after being treated with its CAR-T therapy, followed by a bone marrow transplant.
“This is the first time CAR-T has ever worked in AML,“ CEO Christian Homsy told me. “It is also the first time CAR-T has been found to be effective, in one patient, without the addition of [chemotherapy] preconditioning.“
As Homsy points out, this has been observed in only one of three patients with AML in the trial — the other two responded initially but relapsed after 2 and 4 months. But it still opens the possibility of using CAR-T cells without the need of intensive chemo beforehand. Celyad will be testing the efficacy and safety of its CAR-T therapy with and without preconditioning to determine which one has the best risk-benefit profile.
“We are now running many different Phase 1 trials to look at what are the best conditions to have a higher chance of success,“ says Homsy. “By the end of 2019 we aim to be in a position to start a pivotal trial with AML, like the ZUMA-1 trial was for Kite.“
Celyad is not the only CAR-T developer going after AML — a much bigger indication that those of Kymriah and Yescarta. Cellectis is testing an off-the-shelf version of CAR-T in AML, though its trial was halted temporarily after a patient’s death. Juno Therapeutics has also started a clinical trial in AML.
Besides the AML trials, Homsy expects to have proof of concept data for CYAD-01 in solid tumors — which are especially challenging to target for CAR-T cells — as well as with an off-the-shelf CAR-T that does not require individualized manufacturing. His ultimate goal is to further improve the impact that CAR-T has had in cancer.
“If you think about CD19 CAR-T today, in the context of B-ALL, Kite has one of the best response rates ever, of around 35% objective response at 6 months,“ he told me. “That means that 65% of patients still do not respond; Can we do better than that?“
Images via Shutterstock






