Newsletter Signup - Under Article / In Page
"*" indicates required fields
AstraZeneca’s MedImmune (UK) has published phase Ib study results in The Lancet Oncology. These showed anti-tumour activity of a combination of investigational antibodies in patients with lung cancer.
 The trial considered patients undergoing treatment with durvalumab and tremelimumab, to see whether the drug combo could act therapeuticaly against locally advanced or metastatic non-small cell lung cancer (NSCLC), irrespective of PD-L1 status.
The trial considered patients undergoing treatment with durvalumab and tremelimumab, to see whether the drug combo could act therapeuticaly against locally advanced or metastatic non-small cell lung cancer (NSCLC), irrespective of PD-L1 status.
Less than half of patients with NSCLC have tumours that are PD-L1 positive, leaving a significant unmet medical need in the PD-L1 negative patient population.
A cohort of 26 patients who were PD-L1 positive and PD-L1 negative status were treated (22% and 29% Objective response rate respectively) resulting in an overall 23% response rate for both types.
Durvalumab is an investigational human monoclonal antibody directed against programmed death ligand-1 (PD-L1), which blocks the interactions between PD-L1 and both PD-1 and B7.1 proteins.
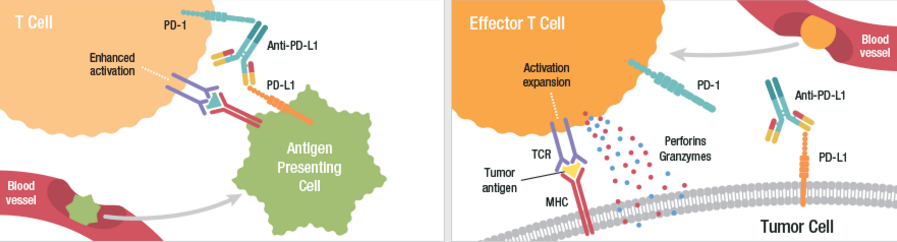
Tremelimumab on the other hand inhibits the activity of cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) to boost the immune response against cancer cells. Last year, tremelimumab was also granted Orphan Drug Designation by the FDA as a potential treatment for malignant mesothelioma.
A preliminary analysis of data from Study 006 was presented at the annual meeting of the Society for Immunotherapy in Cancer (SITC), in November 2015.
The latest findings reinforce MedImmune’s belief that the combination strategy is key to the future success of immuno-oncology treatment, although significant attention needs to be paid to the adverse side effects (with 16% of patients dropping out of the trial)…
UPDATE since Publication on 09/02/2016:
The FDA has put a partial hold on the development of durvalumab in combination with tremelimumab after a number of ‘bleeding events’ unrelated to head and neck cancer were reported. As Endpoints reports, “AstraZeneca can ill afford any delays in the development of durvalumab now,” since its development has weathered a number of setbacks. Following the news of the hold, shares have slid 5%.
An Explanatory Video by AstraZeneca of the PD-L1 Holy Grail in ImmunoOncology
Feature Image Credit: MedImmune Media
Oncology R&D trends and breakthrough innovations






