Newsletter Signup - Under Article / In Page
"*" indicates required fields
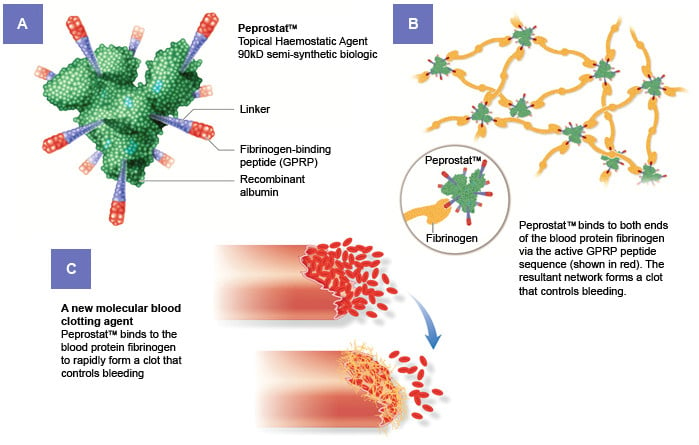
Ergomed has announced positive results in Phase II with PeproStat, a peptide solution to stop bleeding during surgery faster.
In a Phase II study that recruited 169 patients across 16 European hospitals, London-based Ergomed has demonstrated that itspipeline’s lead candidate, PeproStat, can reduce the time to achieve hemostasis, that is, for the bleeding to stop, during surgery as compared to the current standard of care.
Currently, bleeding episodes during surgery are stopped using a saline solution that is applied using a gelatin sponge. Ergomed replaces the saline with a solution that contains PeproStat, a peptide with coagulant activity. The peptide acts by binding fibrinogen to rapidly form a clot that stops the bleeding.
The results of the clinical trial revealed that, PeproStat reduced the average time to hemostasis by 1.55 minutes, from 5.8 minues with saline to 4.2 minutes with PeproStat. Although the reduction might seem small, reducing bleeding by a couple of minutes can be critical during surgery.
“Surgical bleeding is a common problem that can be associated with significant blood loss, increasing patient morbidity and mortality. PeproStat works fast and has shown to reduce the non-responder rate by almost 50%,” stated Paul Hayes, Doctor at the Addenbrookes Hospital in Cambridge and Chief Investigator of the study. “This could be hugely beneficial to patients, reducing operation times, complications and preventing unnecessary returns to surgery.”
Ergomed is planning to start a Phase III trial with PeproStat in 2018, and CEO Dan Weng expects to see the product in the market as soon as 2020. The company estimates that its product could unlock a $2.5Bn (€2Bn) market. This would be a major win for the company, which, though it started off as a CRO, could soon be launching its first product.
Ergomed could be joining a wave of new technologies designed to modernize surgery, such as Gecko Biomedical’s biocompatible surgical glue to reduce bleeding during vascular reconstruction surgery, which recently received approval in Europe.
Images via S_L /Shutterstock; PeproStat