Quality of RNA and viral vectors used in clinical trials is subject to strict regulation. Plasmids that are needed for production of these complex molecules are also on authorities’ radar. German-based biotech PlasmidFactory, delivers the right solution with its High Quality Grade plasmids.
For decades, artificially constructed plasmids have been used as vectors in genetic engineering to clone, amplify or express particular genes. A wide variety of plasmids are commercially available for such uses but not all of them are suitable for a clinical trial with humans.
The EMA (European Medicine Agency) has set up strict guidelines forcing clinical trials to use the highest quality standards. For example, Viral vectors or RNA need to be produced under GMP (Good Manufacturing Practices), a very strict guideline for laboratories. While plasmids don’t need a GMP authorization in these cases, they still need to be High Quality Grade. This quality standard requires highly purified DNA and a strong documentation on the production process.
High Quality Grade plasmids from PlasmidFactory are particularly suitable for GMP-compliant production of viral vectors, RNA, antibodies or, depending on the respective regulation, induced pluripotent stem cells (iPS).

Plasmids offered by PlasmidFactory are the highest quality of plasmid available worldwide. Thanks to a patented purification method involving multiple chromatography steps, PlasmidFactory is able to produce plasmids without any animal derived substances. The High Quality Grade production process is also enzyme and antibiotics free from fermentation on. To prevent cross-contamination, PlasmidFactory produce exclusively one type of plasmid at a given time in a dedicated facility with separate laboratories for fermentation and chromatography, both performed under controlled and reproducible conditions.
Research institutes and companies in Europe and the USA use the High Quality Grade Plasmid DNA produced in Germany by PlasmidFactory to produce RNA and viral vectors used in clinical studies investigating for example, gene therapy.
Use of plasmids in Gene therapy
Recently, gene therapies have gained a new momentum thanks to the development of safe viral vectors based on adeno-associated viruses (AAV).
AAV are preferred to other approaches for gene therapy as they cause no viral disease symptoms in the target organism, and only a mild immune response is to be expected. AAV are also more efficient in term of transfection of cells or tissues.
The pDG plasmids, offered by PlasmidFactory for the production of adeno-associated virus (AAV), form a 2-plasmid system. In contrast to cost intensive triple transfection with three different plasmids, only two components are needed: a plasmid with the gene to be transferred and the corresponding pDG plasmid containing all the information for the propagation and packaging of AAV vectors. Unlike original systems, the pDG plasmid doesn’t need additional infection with helper viruses.
PlasmidFactory has obtained an exclusive, worldwide license for the production, distribution and use of the pDG family from the German Cancer Research Centre (DKFZ). These plasmids are now available to help biotech companies during their clinical trials (find out more about pDG HERE).
PlasmidFactory, a reference on the market
Since its beginning in 2000, PlasmidFactory has provided industrial and academic research scientists with plasmid DNA but also another innovative product: the Minicircle DNA.
Minicircle DNAs contain almost exclusively the “Gene of Interest” and its regulating sequence motifs, allowing an increase of the dose without increasing the amount of DNA.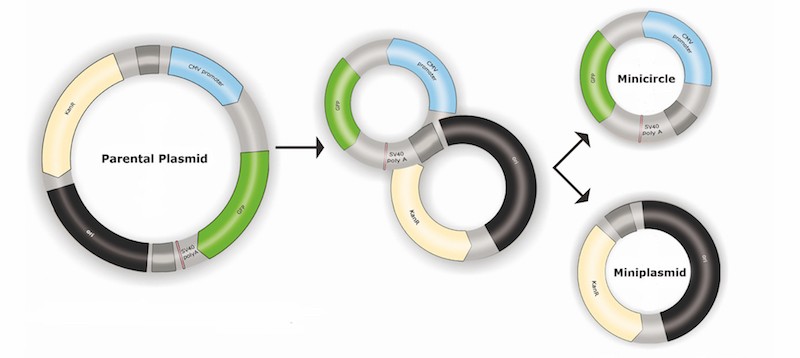
Redundant bacterial backbone sequences are completely removed, resulting in a safe and highly efficient vector system meeting future regulatory requirements for gene therapy and vaccine products (more details about minicircle DNA HERE).
Thanks to PlasmidFactory High Quality Grade plasmids, Biotech companies involved in a clinical trial can now operate with compliance of the regulations. The product can be customized to each need and a strong documentation is always delivered in addition of the plasmid to ensure the traceability of the product.
Find out more about PlasmidFactory HERE






