Newsletter Signup - Under Article / In Page
"*" indicates required fields
Modus Therapeutics closed SEK 140M (€13.5M) in Series A financing to complete a Phase II study testing its sickle cell disease drug and to prepare for further clinical trials.
The financing round was led by HealthCap, a European venture capital firm focused on life science investments, which will contribute SEK 60M (€5.8M). The remaining funds were raised from existing shareholders. Last year, the company raised €3.4M to support the start of the same Phase II trial.
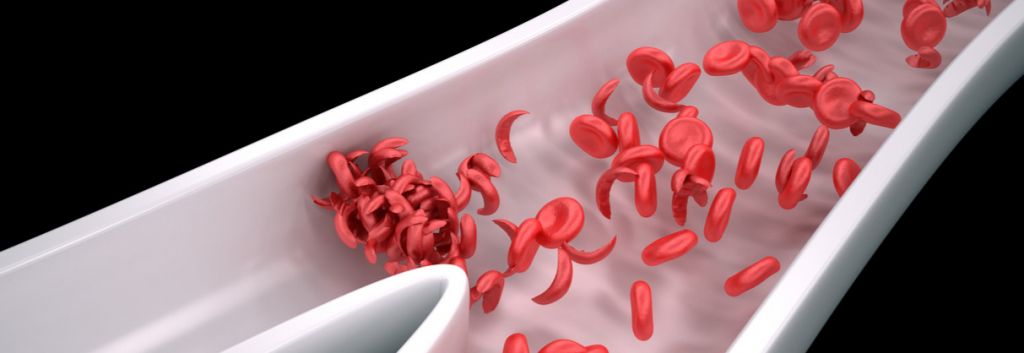
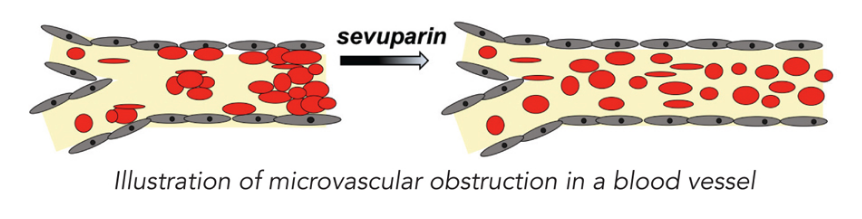
Modus Therapeutics, based in Stockholm, focuses on developing treatments for sickle cell disease and malaria. The company’s lead candidate, sevuparin, is similar to the blood thinner heparin. It has the same anti-inflammatory and anti-adhesive properties of heparin, but does not trigger spontaneous bleeding. The drug has the potential to restore blood flow and treat vaso-occlusive crisis, a potentially lethal complication of sickle cell disease where sickled red blood cells block blood flow in the blood vessels, causing injuries. Modus plans to enrol the last patients in its Phase II study by the end of this year and is also testing sevuparin in malaria, where the drug can normalize blood flow by releasing parasite-infected red blood cells into circulation.
Sickle cell disease affects millions of people worldwide, especially people with ancestors from sub-saharan Africa, South and Central America, Saudi Arabia, India, and the Mediterranean. The disease becomes noticeable in the first years of life, with patients getting painful swellings on their hands and knees, as well as fatigue due to anemia.
Today, stem cell transplantations from bone marrow are the only way to cure the disease, but these can cause serious side effects like graft versus host disease. Luckily, biotechs are working on new alternatives. For example, to take over the development of an antibody treatment for sickle cell disease, Novartis bought US-based Selexys Pharmaceuticals in 2016 based on positive Phase II results. CRISPR Therapeutics and its partner Vertex Pharmaceuticals hope to start a Phase I/II trial testing their CRISPR technology in patients with sickle cell disease very soon, however, the FDA has placed a hold on their application for now.
This leaves Modus still in the race with its European competitors in developing an effective sickle cell treatment. However, US biotech Global Blood Therapeutics is leading the way. The company is confident its positive Phase III results will be enough to bring its sickle cell candidate voxelotor through regulatory approval.
Images by Modus Therapeutics, decade3d – anatomy online/Shutterstock