Newsletter Signup - Under Article / In Page
"*" indicates required fields
Update (13/03/2018): Poxel has started two more Phase III trials in Japan testing its drug for type 2 diabetes, imeglimin.
This oral drug has shown potential to lower glucose levels thanks to a mechanism of action that targets the pancreas, the liver and muscles simultaneously. The new trials will confirm whether these effects are maintained in a larger population of a total of 1,100 patients.
Poxel expects to obtain results in 2019 and apply for approval in Japan by 2020. If successful, approval could then follow in Europe and the US.
Originally published on 04/05/2018
Poxel scored positive results for its type 2 diabetes drug in a Phase IIb study in Japan, preparing the company to go for a Phase III program.
Poxel, one of our top biotechs in Lyon, wants to go all the way, at least in Japan. The company just announced that it achieved its primary and key secondary endpoints in a Phase IIb study in Japan for its type 2 diabetes candidate Imeglimin. Now, the company plans to go for a Phase III program that could be launched later this year.
While the company postponed its plans to file an IPO on Nasdaq last year, the Merck Serono spinout decided instead to use its Euronext listing to pile up €26.5M in cash, allowing the company to fund the recent Phase IIb trial for its lead diabetes candidate.
The drug, Imeglimin, is designed to enhance the bioenergetics of mitochondria, whose dysfunction plays a major role in insulin resistance and diabetes type II. Additionally, the compound has protective effects on beta-cell survival and function and therefore may delay type 2 diabetes progression.
Indeed the drug was able to significantly reduce levels of glycated hemoglobin A1c as well as levels of Fasting Plasma Glucose (FPG), which makes the drug a promising candidate as a monotherapy or as an add-on to other glucose-lowering therapies for the treatment of type 2 diabetes. The results were consistent with what was observed in the US and EU, where Poxel already completed its Phase II programs.
While the French biotech is looking to partner with bigger companies that have the Phase III budgets needed to advance clinical development in the US and the EU, Poxel is making a bold move for a small biotech and plans to go solo in Japan.
“In contrast to the U.S. and Europe, you can progress very quickly and aggressively in Asia,” Thomas Kuhn, CEO of Poxel, commented in an article by FierceBiotech. Kuhn seems to be confident that Poxel can pioneer this on their own.
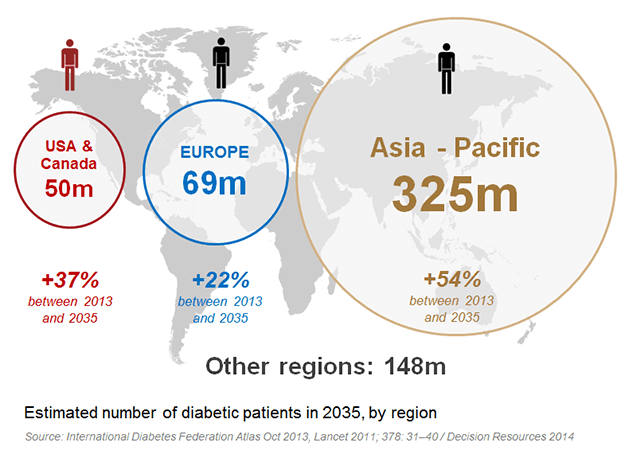
Should the company succeed, it is looking at a highly profitable market. Japan represents the second largest single market for type 2 diabetes, which Poxel expects to grow to approximately $6 billion in annual sales in 2020. While big players like Sanofi, Novo Nordisk and Eli Lilly are all still bidding on GLP-1, Poxel’s unique candidate has good chances to succeed on the crowded market.
Images via shutterstock.com / Maya Kruchankova and poxel.com