Looking to expand your partner network with leading companies like Poxel? Consider joining Inpart's global network for free.
News and Trends 3 Oct 2022
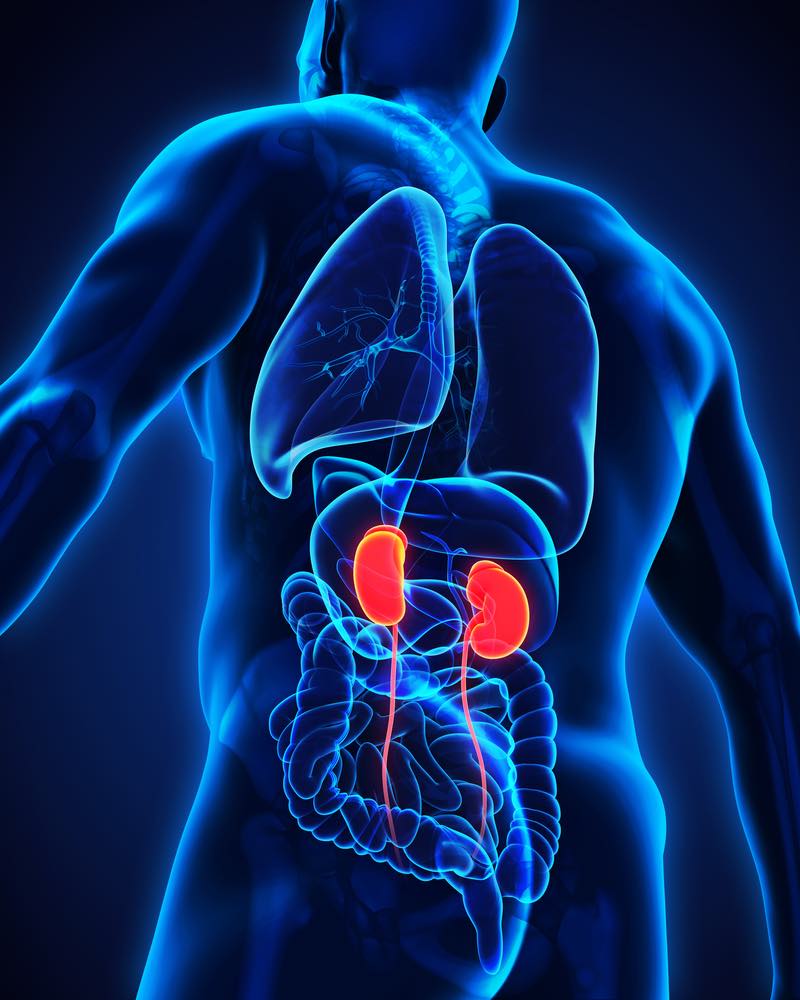
Poxel granted orphan drug designation for kidney disease treatment
French biopharma company Poxel SA says the U.S. Food and Drug Administration (FDA) has granted orphan drug designation (ODD) to PXL770 for the treatment of patients with autosomal-dominant polycystic kidney disease (ADPKD). PXL770 is a novel, first-in-class direct adenosine monophosphate-activated protein kinase (AMPK) activator – and is also a phase 2 ready ADPKD asset, subject […]