Newsletter Signup - Under Article / In Page
"*" indicates required fields
Ewing sarcoma is the second most frequent bone tumor in children, adolescents, and young adults.
There is no specific treatment for this disease and current management is still limited to surgery, radiotherapy, and chemotherapy. The long-term survival of patients with metastatic or relapsed Ewing sarcoma is very low.
Ewing sarcoma is caused by a single oncogene that results from the fusion of two genes. Although a variety of genes may be involved, EWSR1 and FLI1 and the resulting cancer-driving oncogene, EWS-FLI, are found to be responsible in the majority of patients.
Unlike most other types of cancer, all attempts to develop experimental animal models of Ewing sarcoma in mice (expressing the EWS-FLI oncogene) have failed.
Prompted by the need for a genetically tractable model that could be used to study the disease, researchers led by Cayetano González, ICREA research professor at IRB Barcelona, and Jaume Mora, scientific director at the SJD Pediatric Cancer Center Barcelona (PCCB), have engineered Drosophila (a genus of small flies) transgenic strains that express a mutant variant of the human oncogene called EWS-FLIFS.
They have found that expression of the human EWS-FLIFS protein in certain types of Drosophila cells triggers the same oncogenic pathways known to account for EWS-FLI oncogenic activity in human patients.
Lighting up two oncogenic pathways
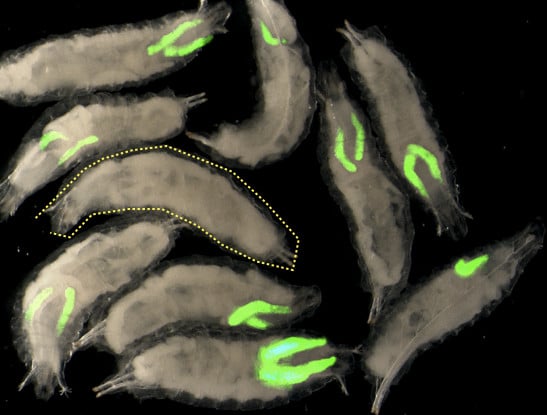
Building upon their new transgenic Drosophila line, the authors rewired two oncogenic pathways used by EWS-FLI, such that when triggered by the presence of EWS-FLIFS, they result in the expression of a fluorescent protein that would otherwise never be expressed. Thus, rather than tumor growth, the researchers use fluorescence as a read-out of EWS-FLI oncogenic activity.
Discovering critical proteins
“This simple genetic trick greatly facilitates the implementation of massive genetic and chemical screens to identify ‘modifiers’ that inhibit EWS-FLI’s oncogenic activity as inhibitors of the appearance of fluorescence,” said Cristina Molnar, a postdoctoral researcher at IRB Barcelona and first author of the study.
Genetic screens based on this new model will make it possible to discover critical proteins required for EWS-FLI to exert its oncogenic function, leading to a better understanding of the molecular basis of the disease, as well as identifying new putative therapeutic targets. Chemical screens may identify compounds that could serve as lead molecules for the development of therapeutic drugs.
The laboratories led by González and Mora have been working together since 2019 to explore the use of Drosophila as a model for pediatric cancer.
Ongoing lines in this collaboration include genetic and chemical screens based on the Ewing sarcoma model and the development of new Drosophila models for other types of pediatric cancer.
Oncology R&D trends and breakthrough innovations