Newsletter Signup - Under Article / In Page
"*" indicates required fields
UPDATE (05/09/2017): Innovate UK has granted Themis £3M (€3.3M) to push its Chikungunya Vaccine towards Phase III.
Originally Published 06/06/2017
Themis Bioscience will test its vaccine candidate against Chikungunya fever in a new Phase I/II trial sponsored by the NIH.
Based in Vienna, Themis Bioscience develops new vaccine candidates for emerging diseases like Zika and Chikungunya with its virus vaccine vector technology, which the biotech licensed from the Institut Pasteur in Paris. Its Chikungunya vaccine is already in Phase II trials in Europe and will now enter a new Phase I/II trial in the US, which is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH).
The NIH’s decision to sponsor the development of Themis’ vaccine candidate was largely based on a recent Phase I trial, where it achieved a 100% seroconversion rate, meaning that all vaccinated subjects produced antibodies against the virus. Now, the new US-based trial will assess the induction of neutralizing antibodies as well as the T-cell responses in up to 180 people.
Chikungunya fever is a viral infection transmitted by mosquitoes, which is marked by severe joint pain accompanied by fever and headache. The pain typically eases after about a week but can persist for months or years in some cases. Today, there is no treatment available for chikungunya infections, and no vaccine exists to prevent it.
Since its appearance in the Western Hemisphere in late 2013, cases of chikungunya have soared, and within the last three years, well over 1.5M cases have been reported in the Americas and the Caribbean alone, highlighting the urgent need for an affordable prophylactic vaccine.
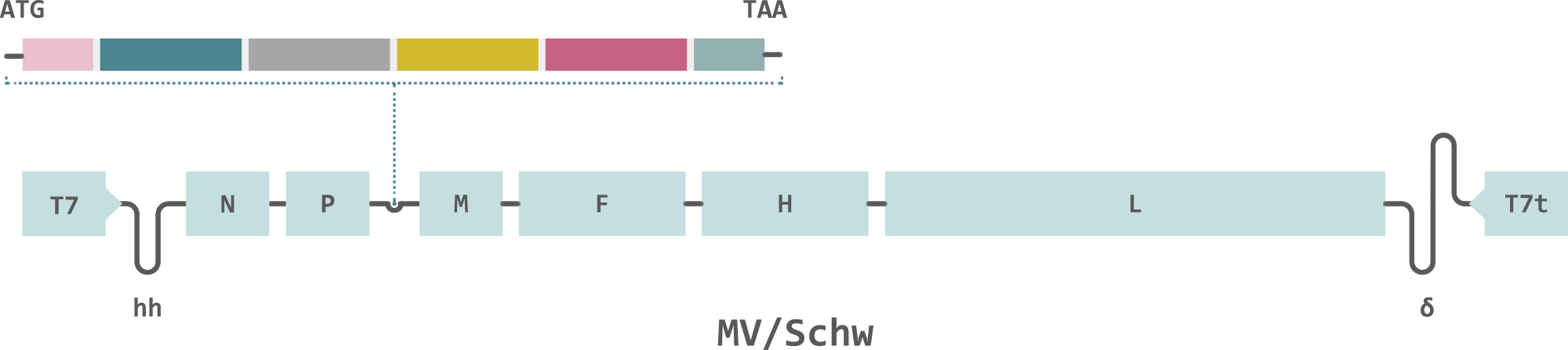
Themis’ vaccine is based on its measles vector Themaxyn platform, where antigens from the Chikungunya virus have been inserted into the well-established measles vaccine, which has proven its superb efficacy and safety profile on over a billion individuals over the last 30–40 years. Further, one of the major advantages of Themis’ Chikungunya vaccine is its cost-efficiency: it can rapidly be scaled up for distribution in the event of an outbreak.
Results of Themis’ Phase II trial in Europe are expected in the second half of 2017. Meanwhile, the NIAID is also testing its own vaccine candidate for chikungunya in an ongoing Phase II trial, while in Europe British Oxitec is directly engineering the Aedes aegypti mosquitoes that transmit the disease to reduce their populations in affected areas. Oxitec has already initiated programs in Brazil, the Cayman Islands and recently India.
Images via shutterstock.com / Nikelser and themisbio.com