Monoclonal antibodies have long been the focus of research surrounding drug development against inflammatory diseases and cancer. For decades, researchers have had to travel along a strenuous path to get to where we are now.
After monoclonal antibodies (mAb) were discovered in the first half of the 20th century, researchers quickly understood that animals, such as mice and rabbits could be used to produce antibodies for human therapeutic use.
Murine monoclonal antibodies were used until the late 1900s. Soon, however, it became apparent that the disadvantages outweighed their positive characteristics: next to a lack of efficacy, the antibodies’ murine origin triggered strong immune responses, so-called human anti-mouse immune (HAMA) responses, in humans.
Circumventing dangerous immune responses
HAMA responses led to allergic reactions with varying severity in treated humans, and sometimes even to life-threatening situations. Consequently, scientists had to admit that something else had to be done.
They began studying ways to reduce and eradicate HAMA responses. Then, in 1988, Greg Winter and his colleagues discovered a way to humanize monoclonal antibodies through antibody phage display – a technique, which allows the study of a protein’s interactions with other proteins, peptides or DNA molecules.
The appearance of transgenic mice

Soon, the first generation of transgenic mice appeared: human antibody genes were inserted into the mouse genome, enabling the animal to produce human antibodies. However, the human body carries a collection of millions of antibodies and early generations of transgenic mice were limited in the amount of antibody sequences they were able to produce, meaning they could only express a small fraction of this antibody diversity.
Then, in 2010, a group of scientists started working on a mouse line that could produce any human antibody in the human antibody repertoire. They founded TRIANNI, inc and began offering the Trianni Mouse™ in 2014.
To date, the Trianni Mouse is the only transgenic antibody discovery platform that offers the entirety of the human antibody gene diversity in a single organism. In essence, antibodies produced by the Trianni Mouse are a perfect match for humans.
What are hybridomas?
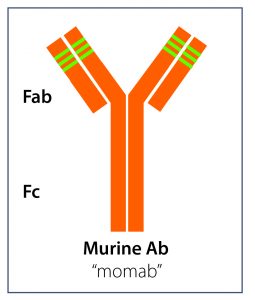
Today we know that antibodies (Ab) or immunoglobulins (Ig) are Y-shaped proteins produced by the immune system’s B-cells when the body is confronted with a pathogen.
The so-called paratope of an antibody is specific to a particular epitope, a binding site that sits on the pathogen’s antigen. In a lock and key mechanism, the antibody’s paratope will lock onto the epitope of the antigen, rendering it harmless or triggering further immune responses.
Although antibodies were discovered much earlier, it wasn’t until 1975 that the researchers Niels Jerne, César Milstein, and George Koehler succeeded in creating so-called hybridomas that could produce antibodies which were specific to the epitopes of certain antigens. It was the hybridoma technology that was used to create the first murine antibodies for human therapeutic use. Today, it is largely outdated.
Hybridomas are cells that can produce large numbers of monoclonal – identical – antibodies (mAb). They are created through the injection of a specific antigen into a mammal, usually a mouse. The B-cells in the animal’s immune system produce antibodies that target the injected antigen. They are then extracted from the animal and fused with specific immortal cancer cells, called myelomas.
The idea behind a hybridoma, is to combine the longevity of the cancer cell with the antibody-production of the B-cell. Hybridoma cell lines can be grown in culture with the great benefit of producing one type of chemically identical antibody per culture.
Problems of accessibility
The emergence of recombinant DNA techniques simplified genetic manipulation for the production of monoclonal antibodies and made it more accurate. Although transgenic mice now included the full human antibody repertoire, companies and scientists alike had severe difficulties in gaining access to them.
So the research team at TRIANNI decided to address this problem of accessibility. Unlike other transgenic mice, the Trianni Mouse contains the exons encoding human antibody diversity, while still keeping the natural mouse control regions in place.
Understanding The Trianni Mouse
How does it work? In the Trianni Mouse, a group of genes called the VDJ genes are deleted and replaced by the complete functional human VDJ gene repertoire. The V genes in that group are chimeric, which means they are part human, part mouse.
Whereas the genes’ non-coding sequences are of mouse origin, the human part of the V genes are the exons. Exons are the encoding part of a gene that will later end up becoming a protein, in this case the antibody.
The combination of human and mouse sequences allows the production of chimeric monoclonal antibodies. Although transgenic Trianni mice have the same antibody repertoire as humans, they still have the natural immune response of the wild-type mouse. So if a Trianni Mouse is confronted with a pathogen, its immune system can still react in the same way as that of a natural mouse.
A large market

Today, monoclonal antibodies represent the largest class of biopharmaceutical products on the market. By 2020, the market for therapeutic antibodies is expected to reach $125 billion (€105 bn).
Monoclonal antibodies are being used for a wide spectrum of applications, such as immune-regulation after organ transplants, or in the development of drugs against inflammation, infection, cancer, cardiovascular-, respiratory-, or ophthalmologic diseases.
The industry has recognized the massive advantages of monoclonal antibodies and is making the best of it. Advantages include: high specificity, supreme safety profiles, and a very fast route of clinical proof-of-concept for targeted therapies.
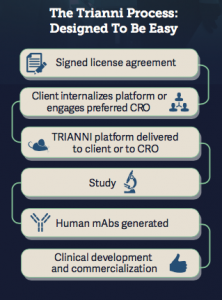
With its transgenic Trianni Mouse, Trianni has created an organism that is capable of expressing any antibody in the human antibody repertoire, making it a valuable tool for drug discovery and development.
The science behind The Trianni Mouse™ is super interesting, but complicated. Check out Trianni’s website for a more detailed explanation, or discover more about its applications and availability here!
Images via Kateryna Kon, Juan Gaertner, Natali_ Mis, Billion Photos, nobeastsofierce/ Shutterstock.com and Trianni, Inc.





