News and Trends 5 Dec 2022
Fallouh Healthcare’s device to give early diagnosis for post-surgery strangulation risk
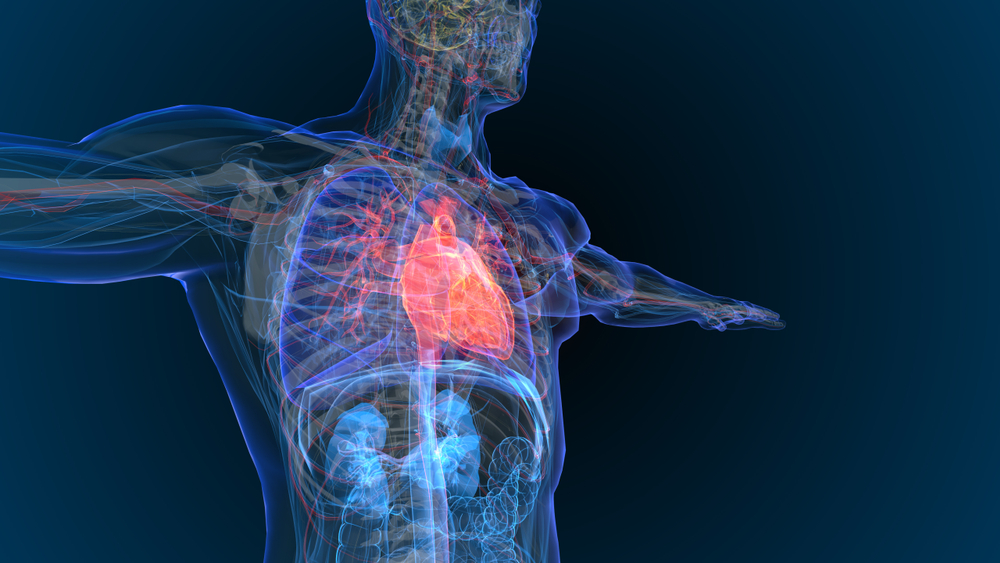
U.K.-based Fallouh Healthcare is developing a device to monitor cardiac patients after surgery, which is when a condition, called cardiac tamponade, can strike. The company says patients recovering from heart surgery can be at risk from the life-threatening condition that sees fluid build-up around the heart, strangling its ability to beat properly. Fallouh Healthcare, formed […]