Newsletter Signup - Under Article / In Page
"*" indicates required fields
Sometimes in biotech, when your standard E. coli just won’t cut it, something more exotic is needed. Could extremophiles be the answer?
Given enough time, and the right mutations, life can adapt to thrive in even the most inhospitable environments. From volcanic hot springs to hyper-saline soils, microbes have made their homes pretty much everywhere. But these ‘extremophiles’ (lovers of extremes) aren’t just fascinating quirks of evolution — their hardiness and unusual abilities have made them increasingly valuable to the world of biotechnology.
Extremophiles have had some notable impacts on modern bioscience. An example is one of the most essential laboratory procedures in molecular biology, polymerase chain reaction (PCR), which enables scientists to make copies of target segments of DNA. This technique employs a heat-stable enzyme called Taq polymerase that was isolated from Thermus aquaticus, a species of bacteria discovered in the hot springs of Yellowstone National Park. For its contribution to science, Taq polymerase was named ‘molecule of the year’ in 1989 by the journal Science.
Since then, scientists have discovered many more of these resilient microorganisms and studied their outstanding abilities. Extremophiles are now part of research projects for all sorts of applications, from agriculture to cosmetics, to dealing with biological hazards.
Fighting chemical weapons
Organophosphorous compounds are infamous for their toxicity. Commonly used as pesticides, they’ve also been turned into highly potent and deadly chemical weapons. These include the nerve agent sarin, deployed in the ongoing Syrian civil war, and VX, used in the 2017 assassination of Kim Jong-un’s exiled half-brother.
Governments across the globe are understandably keen to find new ways of rapidly degrading such toxic compounds. By looking at extremophiles, Gene & Green TK may have found a solution.
The Marseille-based biotech firm was founded in 2013, emerging from research carried out at Aix-Marseille University in collaboration with the French Defence Procurement Agency, with the aim of finding enzymes capable of breaking down organophosphates. The company’s flagship product uses an enzyme discovered in the extremophile archaea Sulfolobus solfataricus, which lives in volcanic pools near Mount Vesuvius.

“This extreme environment conferred a tremendous robustness to the enzyme, allowing it to resist various harsh conditions,” David Daudé, Gene & Green TK’s Chief Scientific and Operating Officer, told me.
“A directed evolution approach was driven in the laboratory to increase its efficiency for the degradation of most organophosphorus compounds in a few minutes.”
The enzyme enables rapid, safe, simple, and environmentally friendly decontamination of organophosphorus weapons and insecticides. It can be incorporated into filtration systems, applied to materials and equipment, and can even be used on the skin in the form of a spray or shower as it is biodegradable and non-toxic.
“It offers a very promising alternative to the conventional chemical solutions, sodium hydroxide and bleach, that are corrosive and not safe for application on humans,” Daudé explained. “Our efforts have led to the development of a decontaminating spray that now needs to be certified according to NATO recommendations.”
Boosting crop health
The global agricultural sector faces the major challenge of maximizing productivity to feed a growing population while reducing dependence on agrochemicals, such as synthetic fertilizers and pesticides, which are increasingly recognized as unsustainable in the long term.
Xtrem Biotech, a spin-out from the University of Granada, is addressing the challenge with agricultural probiotics based on extremophile bacteria. Its extremophile of choice is a strain of bacillus referred to as XT1, originally isolated from hyper-saline soils in southern Spain.
The bacterium has a knack for tolerating difficult conditions, including high soil salinity, drought, extreme pH levels, and exposure to UV radiation. This means that it can survive dormant in the soil for a long time until the conditions are optimal, as well as being easily stored and transported.
But it’s the microbe’s ability to stimulate plant growth and protect against pathogens that makes it a promising alternative to agrochemicals. XT1 produces a range of molecules that promote crop health. These include amino acids and sugars that the plants can use for growth, auxins that increase root formation, and a variety of antimicrobial compounds that can protect against fungi and other plant pathogens.
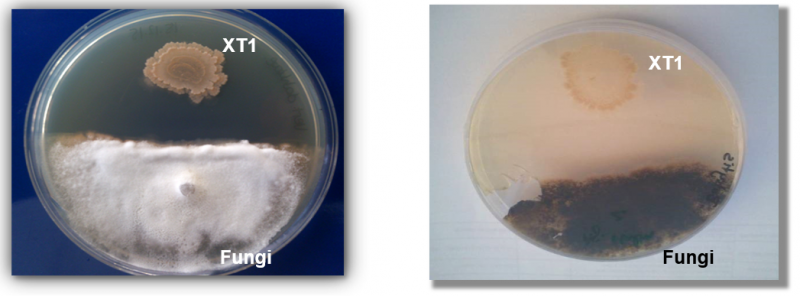
“Typically, efforts to control these problems utilize strong chemicals that follow a ‘scorched earth’ mode of control, killing everything, both good and bad microbes,” said Borja Torres, CEO of Xtrem Biotech.
“The effectiveness of XT1 is similar or better if used correctly. Not in the same way as chemicals but in a preventive way. It is important to underline the sustainability and safety for users and the environment, where biologicals are far ahead of chemicals.”
Xtrem’s biostimulant has already been trialed on tomato, pepper, pumpkin, cucumber, courgette, and strawberry crops, achieving encouraging results.
“After five years of development and field trials, its efficacy is well determined,” Torres explained. “The immediate target on the horizon is to gain a commercial agreement with an established partner to advance in the regulatory process and consider a sublicense of the technology, which will allow us to go further in the development of new products and strains.”
Producing anti-aging molecules
French biotech Deinove has a collection of thousands of species of rare microbes that the company has been putting together since it was founded in 2006. By screening its ever-expanding microbial library, Deinove has singled out several promising extremophiles for a range of applications, including the production of new anti-infective compounds.
Deinove’s most advanced product is an anti-aging agent for the cosmetics industry. The compound is found in an extremophile bacterium called Deinococcus geothermalis, collected from the volcanic hot springs of Réunion Island, a French territory in the Indian Ocean.

“To resist such extreme conditions, they have developed the means to defend themselves. They notably produce carotenoid compounds, which are natural shields. These molecules can be extracted to transfer these resistance properties to skin, which is also facing stress factors like air pollution and UV radiation,” said Emmanuel Petiot, CEO of Deinove.
Phytoene, the original precursor of all carotenoids, has remarkable antioxidant properties, preventing cell degradation and ultimately, skin aging. But humans don’t naturally produce it in our skin, and there wasn’t an effective method of extracting pure phytoene for commercial use. The ability of Deinococcus geothermalis to efficiently synthesize carotenoids — vital for its survival in the wild — made it a prime candidate to produce pure phytoene.
“Deinococcus is a perfect fit, provided you master this bacterium like we do,” said Petiot. “The major challenge was to orient our strain towards hyperproduction of phytoene, in order to provide the first pure highly concentrated phytoene to the market.”
————————
It’s clear that extremophiles are continuing to broaden biotech’s horizons, yet we’ve only seen the smallest snapshot of what nature has to offer. By some estimates, there may be over one trillion microbial species on earth, with 99.999% of them still undiscovered.
Exploring that staggering biodiversity will be a never-ending effort. Probing the most hostile habitats could uncover a wealth of resourceful and tenacious extremophiles whose talents can be harnessed for further applications beyond the capability of most other forms of life.
This is an updated version of an article published by Farhan Mitha on 30/08/2019
Cover illustration by Elena Resko. Pictures via Shutterstock, Xtrem Biotech, and Deinove.
Partnering 2030: FME Industries Report







