Newsletter Signup - Under Article / In Page
"*" indicates required fields
IL-2 cytokine therapy was first approved for kidney cancer in 1992, to stop the spread of cancer cells in patients. But quickly enough, its safety was questioned. Now, a more advanced form of this therapy called IL-2 smart cytokines, or superkines, is being tested in the clinic. They have been designed to circumvent side effects, and could shake up cancer care as we know it.
Interleukin-2 (IL-2) superkines were born out of IL-2 cytokines, after the latter drew criticism for bringing about toxicities in patients. Nevertheless, according to Caroline Loew, chief executive officer of Mural Oncology, cytokines offer incredible potential in cancer research and immunology.
“There are a number of different cytokines, each one with a unique mechanism of action and unique challenge. Thirty years ago, data was published on what is now aldesleukin, a high-dose IL-2, and it was essentially curative in melanoma and renal cell carcinoma (RCC),” said Loew. “This cracked open the world of immuno-oncology research. Unfortunately, aldesleukin’s toxicity profile significantly limited its use.”
The major challenge of treating patients with IL-2 cytokines is that while they can achieve high efficacy by activating cancer-fighting cells like NK cells and CD8 T cells, they also bind to Treg cells, which are meant to suppress the immune system. So, to overcome this, IL-2 superkines, which are essentially engineered cytokines, have been created.
Mural Oncology steers the way with nemvaleukin
American biotech Mural Oncology leads this space with its drug nemvaleukin, which is currently being tested for its safety and efficacy in the clinic.
“We minimally alter the native IL-2 sequence to maintain its potency, and through some very simple but elegant protein engineering block the binding of the alpha receptor, which is what causes the Treg activation and associated toxicity,” said Loew.
Nemvaleukin is immediately active upon dosing and, because it is a stable fusion protein, Loew explained that it does not degrade to native IL-2 and has a longer half-life – the time taken for the active substance in a drug to reduce by half – than IL-2.
“When I saw these extremely deep and durable responses, I was compelled to join the company, and the data gave me deep conviction in our approach.”
The drug has received both orphan drug and fast track designations from the U.S. Food and Drug Administration (FDA) for the treatment of mucosal melanoma, a type of rare and aggressive cancer that begins in the tissues that line organs. It has also gained the fast track designation for platinum resistant ovarian cancer (PROC), in a combination therapy with immunotherapy drug pembrolizumab. PROC is a form of ovarian cancer that needs to be treated even after patients have undergone platinum chemotherapy.
These FDA clearances have allowed nemvaleukin to be evaluated in a phase 1/2 trial for a range of cancers. The drug showed promising responses in combination therapy with pembrolizumab in heavily pretreated patients.
“When I saw these extremely deep and durable responses, I was compelled to join the company, and the data gave me deep conviction in our approach. In PROC, for example, the median duration of response we observed was over 65 weeks compared to standard of care chemo, which is around 15 weeks. That is a significant improvement in response, especially for patients in such a late line of treatment,” said Loew.
Currently, Mural has two studies in place. A phase 3 trial involving 448 patients with PROC, for which final results will be out in 2026, and a phase 2 trial comprising 90 patients with mucosal melanoma. The results will be reported next year. As part of this phase 2 trial, patients with cutaneous melanoma, a kind of skin cancer that originates in the pigment-making cells of the skin, have been put on a new dose. This regimen is a shift from five daily infusions to two infusions, for a three-week cycle.
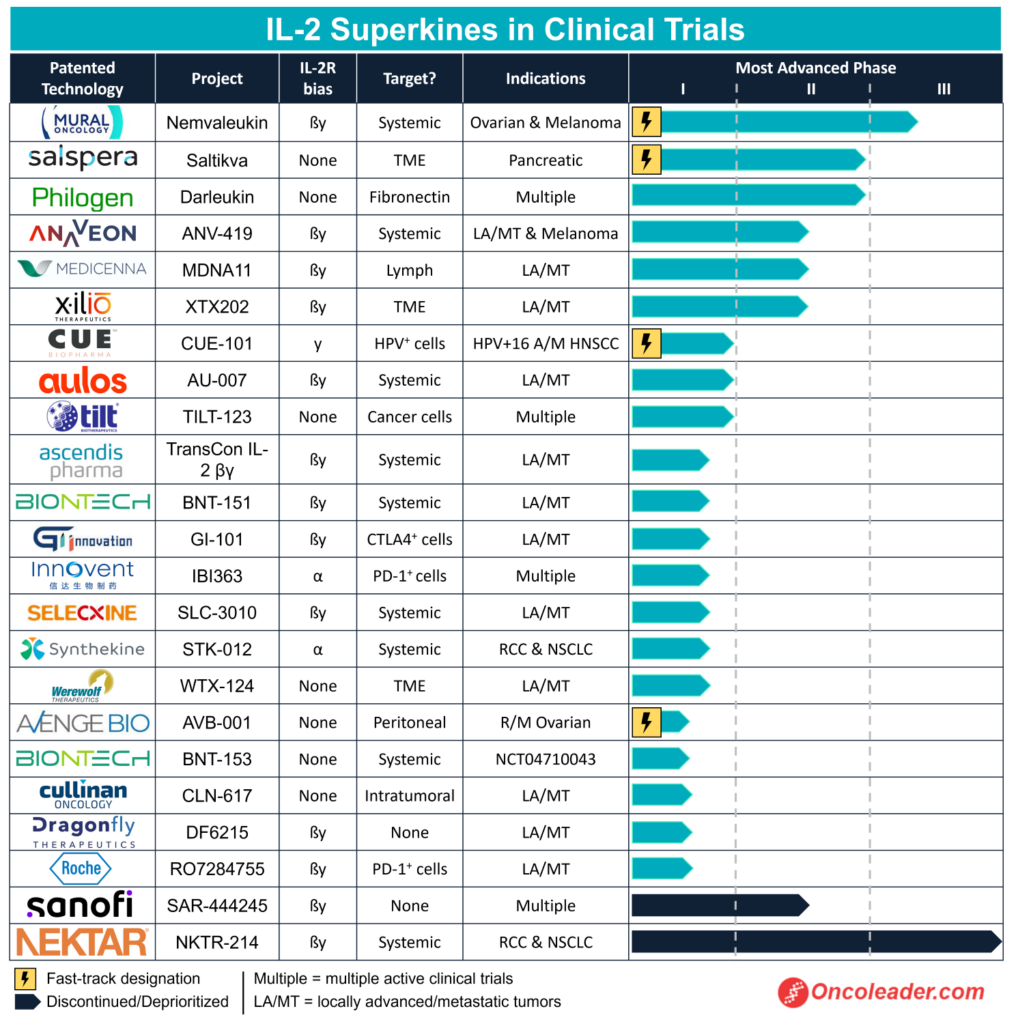
IL-2 smart cytokines in clinical trials
Meanwhile, Mural isn’t the only biotech engineering IL-2 cytokines to target cancer cells. U.S.-based Salspera’s Saltikva is a phase 2 candidate that is on a mission to treat pancreatic cancer. Saltikva is an oral drug that contains an attenuated strain of Salmonella Typhimurium that expresses IL-2. An attenuated organism can be engineered to carry antigens that elicit responses and target disease-causing cells.
The candidate, which was granted fast track designation from the FDA back in 2022, increased the survival of patients with stage 4 pancreatic cancer when administered in combination with chemotherapy. In fact, the regimen showed a median survival of 24 months, which is more than double when compared to the standard of care chemotherapy.
Also in the race to get its IL-2 superkine to the market is Swiss-Italian biotech Philogen. Its clinical feat Darleukin is made up of the L19 antibody, which is fused to a human IL-2 cytokine. It is a targeted therapy that is designed to attack tumor cells and spare healthy ones because it is able to activate immune T cells and NK cells at the site of disease. At present, Darleukin is in a phase 2 trial as it is being tested along with an intense radiotherapy, to improve the progression-free survival in patients with non-small cell lung cancer (NSCLC).
Moreover, Swiss biopharma Anaveon, Canadian company Medicenna, and Massachusetts-based Xilio Therapeutics, are all holding phase 2 trials for their IL-2 smart cytokines as well. Anaveon’s ANV419, like Darleukin, is a protein composed of IL-2 and an anti-IL-2 monoclonal antibody fused together to prevent the binding of the IL-2 to its receptor. It signals through the subunit called IL-2R beta/gamma, which is present on the tumor-fighting immune cells. It is being tested in combination with the monoclonal antibody ipilimumab in patients with advanced solid tumors.
Medicenna’s superkine MDNA11, has been fused with human recombinant albumin, a type of protein that is made by the liver. It is being tested to fight solid tumors, just like Xilio’s XTX202.

Technologies related to cytokine therapy
- Photocaged Cytokines for Cancer Immunotherapy – Emory University
- NUDRA® – A Treatment for Peripheral Neuropathic Pain – OLYS Pharma
Big pharma interest in IL-2 superkines
With many more drug candidates in the clinic, like Massachusetts-based Cue Biopharma’s CUE-101 and California-based Aulos Bioscience’s AU-007, big pharma has also taken a shine to IL-2 superkines.
Swiss multinational Roche is among these pharma giants. It has organized a phase 1a/1b trial to evaluate the tumor-killing abilities of RO7284755. The candidate is being studied as a monotherapy and in combination with monoclonal antibody atezolizumab in patients with solid tumors.
Besides Roche, German company BioNTech is also a key player in the field. In fact, it has two IL-2 smart cytokine candidates in the running. Its phase 1 candidate BNT151 is being developed to treat multiple solid tumors including head and neck cancer, liver cancer, and breast cancer. It is based on its RiboCytokines platform, where the cytokines are encoded by mRNA and produced in the patient. Through this method, BioNTech aims to defeat drug resistance and boost the effect of vaccinations. Its other candidate BNT153 is another product of the platform aimed at targeting multiple tumors.
However, not all pharma giants have met fruitful outcomes. French multinational Sanofi pulled the plug on its phase 2 candidate after it failed the efficacy trial, almost two years ago. And so did NKTR-214, a superkine that arose from a collaboration between American multinational Bristol-Myers Squibb and American biopharma Nektar Therapeutics. The drug was binned after it failed to produce compelling clinical data.
IL-2 superkines in clinic bring hope
But Loew seems confident that Mural’s nemvaleukin is here to stay. As there is an enormous unmet need for people with cancers like mucosal melanoma, calls for more treatment options are getting louder.
“It (mucosal melanoma) is notorious for the low 5-year survival rate of less than 25% (versus cutaneous melanoma: 80%). Progression-free survival varies in studies from only 3 to 4 months. These outcomes are even worse in the checkpoint-inhibitor-experienced mucosal melanoma population,” said Loew, who believes that IL-2 superkines could improve these statistics.
As this revamped class of drugs seems to be moving ahead in the clinic, it will be soon when we finally find out if they were worth the wait after all.
Oncology R&D trends and breakthrough innovations