Newsletter Signup - Under Article / In Page
"*" indicates required fields
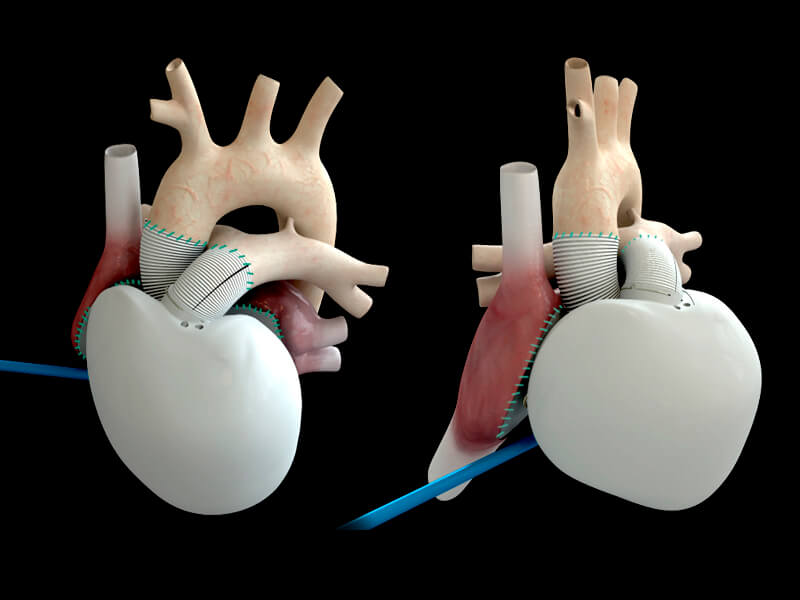
Carmat’s artificial heart is being tested in patients with heart failure. A hospital in Copenhagen will implant the device, which the biotech hopes will boost patient enrolment to its PIVOTAL trial.
Carmat has developed the world’s most advanced fully artificial heart, which has been designed for patients suffering from end-stage biventricular heart failure. The biotech has been given permission to implant its artificial heart into patients at the Heart Center of Copenhagen’s Rigshospitalet Hospital as part of its PIVOTAL study by France‘s National Agency for Security and Medicine. This follows similar decisions at centers in Astana, Kazakhstan, and Prague, the Czech Republic, which has given the company’s stock price a boost of over 2%.
Carmat’s heart offers a potential cure for heart failure, which is a condition characterized by the heart no longer being able to carry out its function pumping blood to all of the cells in the body. The artificial heart is powered by a portable power supply that tells the patient when to return home to recharge it. The biotech believes that its device will be compatible with the blood and body, and reliably pump blood in response to the patient’s physiological needs.
Carmat’s development has been a mix of highs and lows, with the technology generating a lot of excitement, highlighted by big fundraisings, including €50M from private investors. Unfortunately, its progress was slowed by patients receiving the heart not surviving for long – the first of which passed away after just 75 days. This forced the National Agency for Security and Medicine to ban Carmat from carrying out trials for 6 months until guarantees could be made for the heart’s safety.
However, with the ban lifted in May 2017, Carmat has quickly sought to make up for lost time. The company’s CEO, Stéphane Piat, set out the biotech’s plans: “We continue to pursue our strategy of creating a pool of highly specialized centers particularly recognized for their know-how in LVAD studies and we expect to include three more centers in the upcoming months. This should allow us to sustain the patient enrollment pace in line with our objective of finalizing the PIVOTAL study by the end of this year.”
With the heart unable to repair or replace the cells that die during a heart attack, which can lead to heart failure, new approaches are needed to help the 49 million people living with cardiovascular diseases in Europe alone. Regenerative medicine has been put forward as a solution but, so far, progress in the field has been slow. However, Belgian biotech, Celyad, has developed a stem cell-based therapy for heart failure which received Fast Track Designation from the FDA.
Celyad’s C-Cure technology could only help a subpopulation of Phase III study participants, and Carmat will hope that its artificial heart will be able to treat a higher proportion of heart failure patients. The biotech’s main competition will come from other heart transplant technologies, which may be far less sophisticated but also much more affordable.
Images – Dim Dimich / shutterstock.com; Carmat