Newsletter Signup - Under Article / In Page
"*" indicates required fields
We had the opportunity to talk with Roman Hovorka, from The University of Cambridge, who’s working on a revolutionary medical device that can automatically improve glucose levels in people with type 1 diabetes.

An artificial pancreas is a device that measures glucose levels and responds by injecting the right amount of insulin required at any moment in a person with type 1 diabetes. This futuristic concept is actually not so far from reality: the FDA recently approved Medtronic’s MiniMed 670G, the first such automated device for type 1 diabetes. However, although it is a huge advancement, the system is still not fully automatic, which means that the user needs to control insulin delivery manually after every meal.
Roman Hovorka, from The University of Cambridge, is already working on a better version: his research focuses on developing an algorithm that can predict glucose levels in any situation. In a chat with us, he explained this cutting-edge technology.
How do you build a fully automated artificial pancreas?
It can be easily be built from parts already available on the market: a subcutaneous sensor that measures blood glucose every 1-5 min and an insulin pump that delivers the desired amount of the hormone at the right time.
However, calculating the right amount can be a big challenge since insulin requirements vary up to 30% for the same person within a single day. Roman’s research aims to develop a completely automated system, also known as a fully-closed loop, that can predict glucose levels in the next 2-3 hours and deliver insulin accordingly.
The most important part is developing the algorithm. We use model predictive control: inside the algorithm, there’s a computer model of glucose regulation and how it is affected by insulin delivery, meal intake, exercise, and also a large amount of unexplained variability. The model is individualized based on past measurements.”

Does the device provide a better quality of life?
Automatic insulin control is not just a matter of comfort: a system that can make decisions for you can succeed where humans are still struggling.
Type 1 diabetes is very different from your standard disease. Insulin requirements vary greatly from one day to another and there is no way patients can know what they need.”
Irregular blood-glucose levels can have serious long-term complications that include blindness, diabetic foot and heart disease. Therefore, health systems will be interested in offering reimbursement. In fact, insulin pumps have been reimbursed in the past, opening up the path for related technologies. However, the artificial pancreas will have to prove first that glucose levels are indeed improved in the long term.
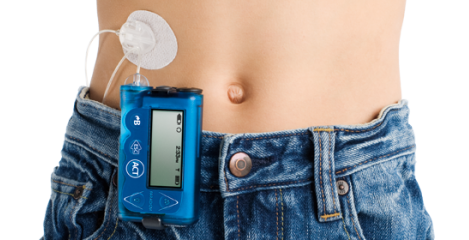
What are the challenges?
One of the main challenges for the development of a completely automated device is accounting for day-to-day variability. To address this, Roman’s group will soon be running longer clinical trials of up to 2 years with the aim to collect enough data to predict the variability reliably.
Another major challenge is insulin delivery:
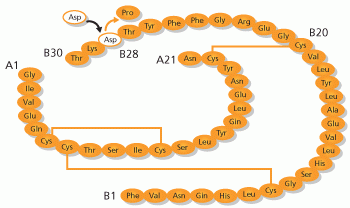
My view is that the delays in the system are mainly due to the absorption of insulin, which usually takes around 60 minutes to peak in the blood.”
But Biotech is already working on it! EliLilly’s BioChaperone Lispro, in Phase III, could reduce the peak time to 42 min. Meanwhile, Biodel is working in BIOD-123, an insulin analog that could reduce the time to around 20 min and has completed Phase II.
When will the technology reach the market?
I think what we currently have could be commercialized. Our current approach is to license it to established manufacturers with existing devices.”
Roman has collaborated with Abbot Diabetes Care, Medtronic, Dexcom and Animas in the past as a consultant, so he has plenty of options to license the technology.
A new technology takes time to establish itself, and not everyone will use the artificial pancreas. Nevertheless, Roman reckons that for those with difficulties to control glucose levels, especially in children and during pregnancies, the technology would be readily accepted.
It is so exciting that after one or two decades of work we finally have a product that has been approved by the FDA… and in only 3 months, which is unprecedented.”
At the very least, Medtronic has opened the door for future developments. If it succeeds, a fully automated artificial pancreas could reach the market within the next 5-10 years thanks to the work of dedicated researchers like Roman. The UK is a leader in healthcare innovation, and its golden triangle is a thriving hub for innovation in biotech.
Images via The University of Cambridge; Click and Photo/Shutterstock; Medtronic; Novo Nordisk