The Spanish Agency of Medicines and Medical Products (AEMPS) has approved the CARxALL clinical trial led by the company OneChain Immunotherapeutics (OCI).
It will be the world’s first trial with CAR-T technology for patients with a subtype of T-cell leukemia.
The CARxALL trial will evaluate, for the first time, the CAR-T CD1a (OC-1) therapy. This treatment specifically targets a subtype of T-cell leukemia, cortical T-cell acute lymphoblastic leukemia (coT-ALL), which accounts for 30 to 40% of T-ALLs and has a poor prognosis in patients who do not respond to currently available treatments.
The clinical production of the CD1a CAR-T therapy for the trial has been led by Maria Castellà, a doctor in the immunology service at the Biomedical Diagnostic Centre of Hospital Clínic Barcelona, which is directed by Manel Juan.
The trial will be conducted at Hospital Clínic and Hospital Sant Joan de Déu in Barcelona, under the direction of Núria Martínez and Susana Rives, principal investigators for the study.
It is expected to include both pediatric and adult patients without any therapeutic alternatives. Although the patients will be treated in Barcelona, patients from Spain and across Europe may join the trial.
The group led by Dr Menéndez, founder of OneChain Immunotherapeutics and director of the Josep Carreras Institute’s stem cell biology, developmental leukemia and immunotherapy group, was the first in the world to develop and validate a CD1a-specific CAR-T for coT-ALL. The study, led by Diego Sánchez and published in the journals Blood and the Journal for ImmunoTherapy of Cancer, has been conducted with animal models using cell lines derived from patients with coT-ALL. Preliminary results show these CAR-T cells persist in vivo in the long term and retain their anti-leukemia activity.
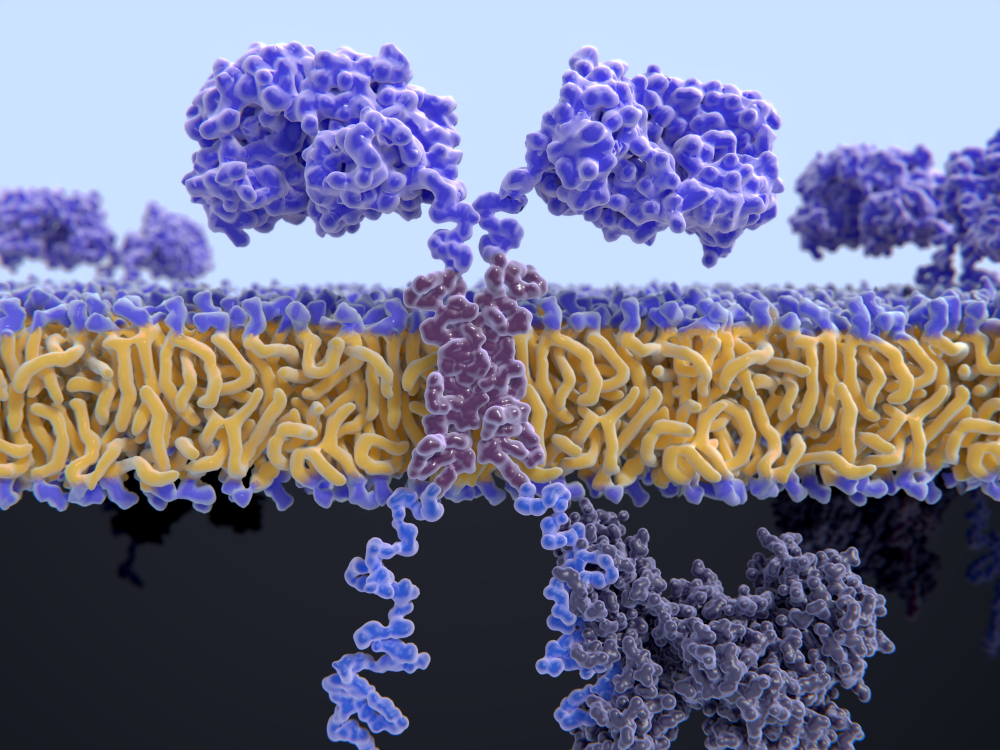
CAR-T cell therapy
The CARxALL clinical trial represents another step towards developing adoptive cell immunotherapy, such as CAR–T cell therapy, a treatment that consists of extracting a patient’s T cells (cells that protect the body from infection), modifying them in the laboratory and returning them to the patient. This modification enables the cells to attack and eliminate the receptors located in the membranes of tumor cells. With this technique, the patient’s own modified cells attack the cancer cells in a directed way, without damaging healthy cells.
OneChain Immunotherapeutics achieved this milestone after being created in 2020 through a round of seed capital amounting to €3.05 million provided by Invivo Ventures, CDTI-Innvierte and the Josep Carreras Foundation, and after receiving funding from the State Research Agency.
Menéndez said: “Developing these strategies and managing all the regulatory mechanisms associated with developing a product is extremely complicated in the academic field. Therefore, OneChain Immunotherapeutics will enable us to carry out all the necessary steps to ensure that our knowledge benefits patients.”
Thymic cortical T-cell acute lymphoblastic leukemia
In Spain, more than 350 children are diagnosed with leukemia every year. Leukemia accounts for around a third of the childhood neoplasms that also occur in adults.
Childhood leukemia is an aggressive type of blood cancer. The affected cells can be of the lymphoid or myeloid lineage. The most common type of leukemia in children is of lymphoid origin and, of these cases, the most frequent kind is acute lymphoblastic leukemia (ALL), which accounts for approximately 80% of childhood cases.
In turn, acute lymphoblastic leukemia can affect the B or T lymphocytes. Around 80% of cases in children are type B.
Just eight out of 10 children manage to overcome the disease. In the case of T-type ALL, fewer than 100 cases are diagnosed each year in Spain. There are several types, depending on the stage of differentiation and mutations.
Cortical T-cell acute lymphoblastic leukemia (coT-ALL) is the most common subtype and accounts for 30-40% of cases of T-ALL. Fewer than 30 children are diagnosed with this disease every year in Spain. They are usually between the ages of 11 and 17. Patients who do not respond to existing therapies (5-15%) may benefit from this immunotherapy.