Painstaking and expensive procedures are required to produce human cells for use in research and cell therapies. Investors are seeking stakes in cell reprogramming startups that can free up the bottleneck, such as Mogrify, bit.bio, and Asgard Therapeutics.
The concept of cell reprogramming — converting one type of cell into another — has been hot on the headlines in the last year.
The UK startup Mogrify led the way in May when it added another €15M to a €15M Series A financing first announced in 2019. Mogrify specializes in producing cell therapies in addition to reprogramming cells in the body to tackle a form of blindness called retinal degeneration.
The UK firm bit.bio raised €88.9M ($103M) in a Series B round earlier this month to finance the development of technology converting stem cells into cells for use in research and disease modeling. And the Swedish upstart Asgard Therapeutics last week raised €6M in a seed round to advance a cancer immunotherapy based on reprogramming cancer cells in the body.
“Our fundraise is amongst the largest ever Series B [rounds for] a UK life sciences company,” said Mark Kotter, CEO and founder of bit.bio. “This demonstrates the strong confidence investors have in the potential of our cell coding technology.”
High investor interest is the result of growing industry prowess in shaping how human stem cells develop in the lab, making them turn into a range of cells including cardiac muscle, skin cells, or nerve cells. Applications for this knowledge include creating disease models, cell therapies, tissue engineering, and more.
The field of cell reprogramming received a major boost in 2006, when a team led by Japanese researcher Shinya Yamanaka discovered a combination of genes that, once activated, converted adult cells called fibroblasts into induced pluripotent stem cells (iPSCs), stem cells that can become any cell type. Yamanaka’s work led to him sharing the Nobel Prize in Physiology or Medicine in 2012.
“Commercial exploration and interest from investors took some time to pick up,” said Cristiana Pires, co-founder and CEO of Asgard Therapeutics. Fifteen years on, the fundraising successes of Asgard and other companies demonstrate the tide is changing.
Pires also noted that discussions with venture capital investors and potential pharma partners about cell reprogramming now reveal “a growing interest in the field and even a fear of missing out on the revolutionary approach.”
Slow and inefficient cell manufacturing techniques hamper traditional life sciences research and cell therapy development. For example, approved CAR-T cell therapies like Yescarta and Kymriah depend on harvesting and genetically engineering the patient’s own T cells. This results in obstacles in obtaining enough cells and transporting them between centers, leading to long waits and high prices for the cell therapy.
Meanwhile, making disease models for drug discovery is complicated by the fact that some adult human cell types are hard to source and use in the lab, such as neurons. Many traditional cell lines used in drug assays have been artificially grown for decades, and can differ widely from cells in the human body. This makes preclinical research hard to replicate and translate to human studies.
The use of stem cells can tackle these problems. Instead of trying to source scarce adult cells, researchers could simply turn plentiful cells like fibroblasts into iPSCs and, from there, grow them into the cell they need.
In order to turn iPSCs into mature cells, researchers traditionally bathe the cells in chemicals such as cytokines in an imitation of how the cells would develop in an embryo. This can be a slow, tedious, and expensive process.
“Essentially, all the cells are made by hand, by highly trained scientists sitting in a clean room,” Nabiha Saklayen, CEO of the US cell therapy developer Cellino Biotech, told Nature this year. “That’s not scalable.”
Many startups aim to solve the scalability problem in cell therapy with techniques including automation and cell encapsulation. However, Yamanaka’s discovery in 2006 also opened the way for cell reprogramming. Rather than mimicking the slow natural process of embryogenesis, researchers could trigger specific genes in cells to change how they grow, making the process faster.
Cell reprogramming involves the activation of transcription factors: proteins that direct which genes should be turned into proteins. Each cell type requires a precise combination of transcription factors, and for a long time, these recipes have been painstakingly researched using trial-and-error approaches.
“The bottleneck here is the identification of the transcriptional regulators able to induce specific conversion into the desired cell type,” said Pires. “The combinations of inducing factors to generate neurons, cardiomyocytes, and hepatocytes have been identified but for most cell types, these cocktails are still unknown.”
Mogrify, Asgard Therapeutics, and bit.bio are part of a thriving group of firms on the cutting edge of cell reprogramming. They use cell banks, RNA sequencing, computational biology, and other resources to decipher the transcription factor combinations that lead to developing a specific cell line for use in cell therapies.
“I believe that European companies are actually spearheading the process, with Asgard being based in Sweden and UK-based companies like Mogrify and bit.bio,” said Pires. “GC Therapeutics is based in the US, as well as several of the companies exploring iPSC differentiation protocol.”
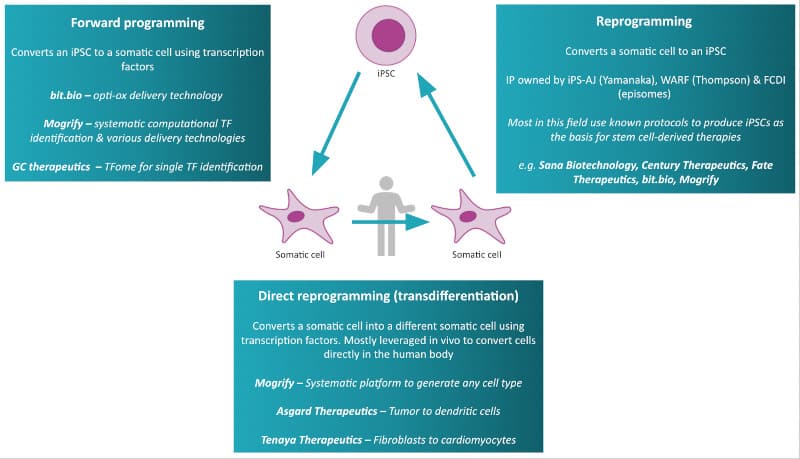
One form of cell technology being honed by bit.bio is forward programming. This technique lets researchers convert iPSCs into mature, or ‘somatic’ cells such as neurons and muscle cells faster and with a higher success rate than traditional methods.
“Reprogramming is entirely dependent on our ability to control the expression of reprogramming factors,” said Kotter. “This is where [our technology] comes into play: it enables highly controlled activation of genetic programs in a cell, similar to the ‘enter’ button on a computer — once you activate it, iPSCs deterministically convert into a new cell type.”
Direct cell reprogramming is another emerging technology opening up many avenues for cell and gene therapy. This process, in development by companies including Mogrify and GC Therapeutics, allows researchers to transform a somatic cell into another type of somatic cell without needing to turn them into iPSCs first. This advantage cuts out many labor-intensive steps from the process and speeds up cell production.
Another tantalizing possibility is the direct reprogramming of cells in the patient’s body (in vivo). This completely circumvents the logistical obstacles of manufacturing cell therapies in the lab.
“Direct in vivo cell reprogramming approaches, akin to gene therapy, could represent the ultimate expression of cell reprogramming’s potential in the conversion of cells for therapeutic effect directly in patients,” said Mogrify’s CSO, Louise Modis. “This represents an entirely new frontier for therapeutic development and a first-in-class approach.”
Mogrify and Asgard Therapeutics are working on making this technology a reality. Asgard, for instance, is developing a cancer immunotherapy that reprograms cancer cells into immune dendritic cells, a type of cell that flags cancer signals to the immune system. This makes the cancer more vulnerable to immune attack.
Reprogramming technology is still at a nascent stage, with many formidable challenges to overcome. For example, cell therapies reprogrammed in the lab need to be delivered to their target sites in the body. Meanwhile, reprogramming cells in vivo makes it hard for researchers to track whether the process is working properly. It’s therefore crucial to have the right transcription factor recipe from the start.
“Most reprogramming processes are still not 100% efficient and some cells retain traits associated with the cell of origin, for example,” said Pires. The company is close to having its reprogrammed cells fully resemble their ‘natural’ counterparts.
The opportunities for those that crack the reprogramming code are myriad. With greater understanding of cell development could come better disease models in drug discovery. Cell types on offer could become ever more accurate and precise, leading to cheaper and more effective cell therapies for cancer and conditions associated with the aging process.
“Cell reprogramming represents a paradigm shift in the way we view biology,” said Kotter. “To me, cell reprogramming is a prototypical example of synthetic biology, which basically means taking a ‘software’ approach to biology. It takes time for tectonic shifts like these to occur and become more mainstream.”
Cover image via Elena Resko. Inline schematic via Mogrify





