Newsletter Signup - Under Article / In Page
"*" indicates required fields
ADCs are overcoming the challenges of first generations and returning with a vengeance. I attended the World ADC Conference to hear about current efforts.
ADCs represent an exciting new field that has seen renewed interest in the past few months, as funding has poured into NBE Therapeutics and ADC Therapeutics. Theses drugs are overcoming the challenges that held the first two generations back to return with a vengeance. Jan Schmidt-Brand, CEO/CFO of Wilex Therapeutics extolls them, “ADCs combine the best of two modalities, toxic efficacy and antibody specificity. The result is an improved therapeutic window with fewer side effects.”
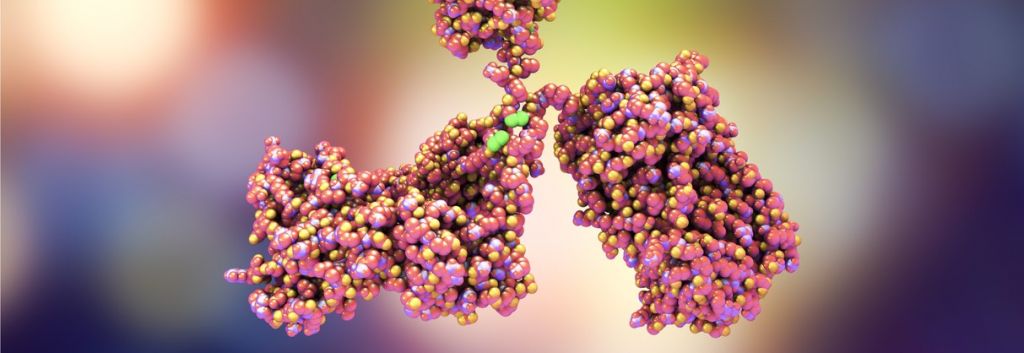
Chris Martin, CEO of ADC Therapeutics, explained to me that an ADC is “a guided missile, where the antibody is the guiding system of a cytotoxic payload.” In an ADC, a toxin piggybacks on an antibody as it homes in on its target receptor protein. The antibody sticks to the cell surface and is then internalized, payload and all, so the toxin can be released inside the cell to induce apoptosis.
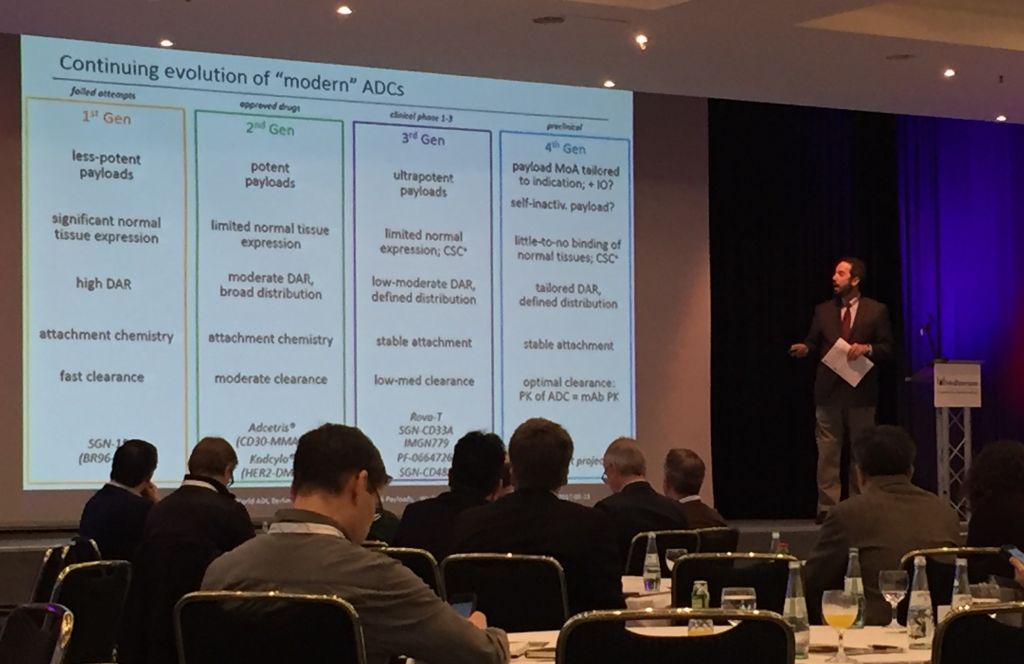
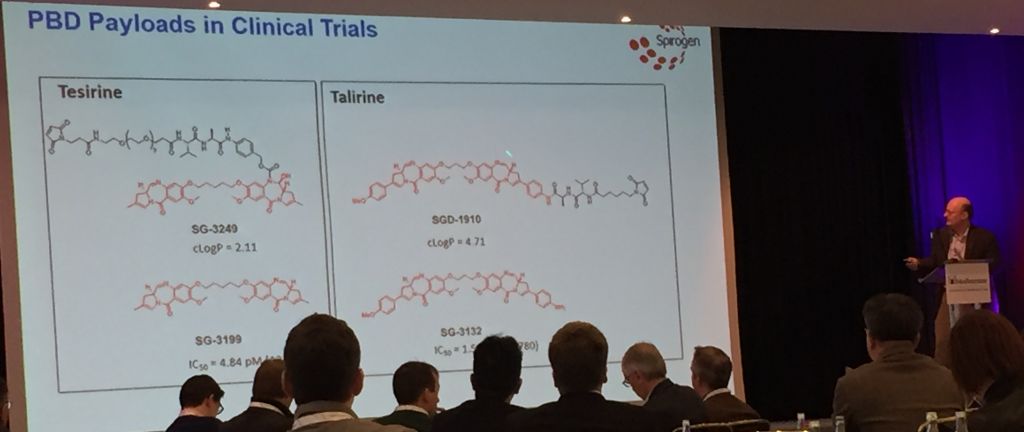
Toxin development has come quite far, since many of the toxins draw on traditional chemotherapies. UK-based Spirogen, which was originally founded by Martin and has since been acquired by AstraZeneca for €416M, is collaborating with Seattle Genetics to develop pyrrolobenzodiazepine (PBD) dimer toxins. These molecules “block cell division without being caught by DNA repair mechanisms. This allows for long-term treatments,” Martin told us last fall.
In his talk at the World ADC Conference this week, Spirogen’s CSO, Phil Howard, went into more detail: the dimers can be linked in a variety of ways, but a particularly instructive study focused on purely carbon chains. Dimers joined with five carbons give the molecule just the right amount of flexibility to wrap themselves into the minor groove of DNA. The optimally snug fit makes Tesirine the most effective cross-linking agent that is thereby able to damage DNA and induce cell death.
However, a much more significant chemistry challenge in ADC development is linker stability. Unstable linkers release the payload too early, which became the critical failure of the first generation of ADC: as a result of ‘de-drugging,’ the first of ADC, Mylotarg, had to be withdrawn from the market following patient deaths.
Alain Beck, Senior Director of Physio-Chemistry at Pierre Fabre, noted that most failures continue to occur for exactly this reason in Phase I. Unfortunately, payload discharge remains somewhat of a mystery — Andrew Bessire, a scientist at Pfizer, described it as a “black box” in his subsequent talk. Many of the research efforts described at the conference were focused on linker stability.
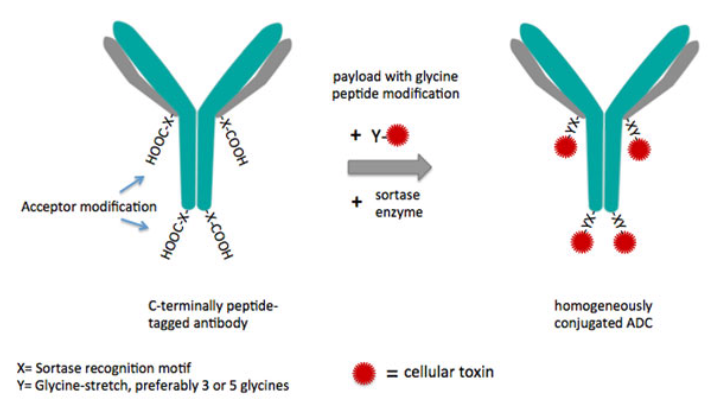
NBE Therapeutics is attempting to perfect the linker’s sensitivity to solvent by substituting the traditional but relatively unstable maleimide linker with a chain of glycines, whose amide bonds are comparatively stable in serum. Indeed, its CEO and founder, Ulf Grawunder showed that “there was no ‘de-drugging’ in mice.” Further, NBE’s ADCs “induced an immunological therapy after two doses of treatment,” meaning the mice did not relapse. Perhaps it was these promising results that persuaded investors to help the company close a €25.8M Series B round of fundraising last fall!
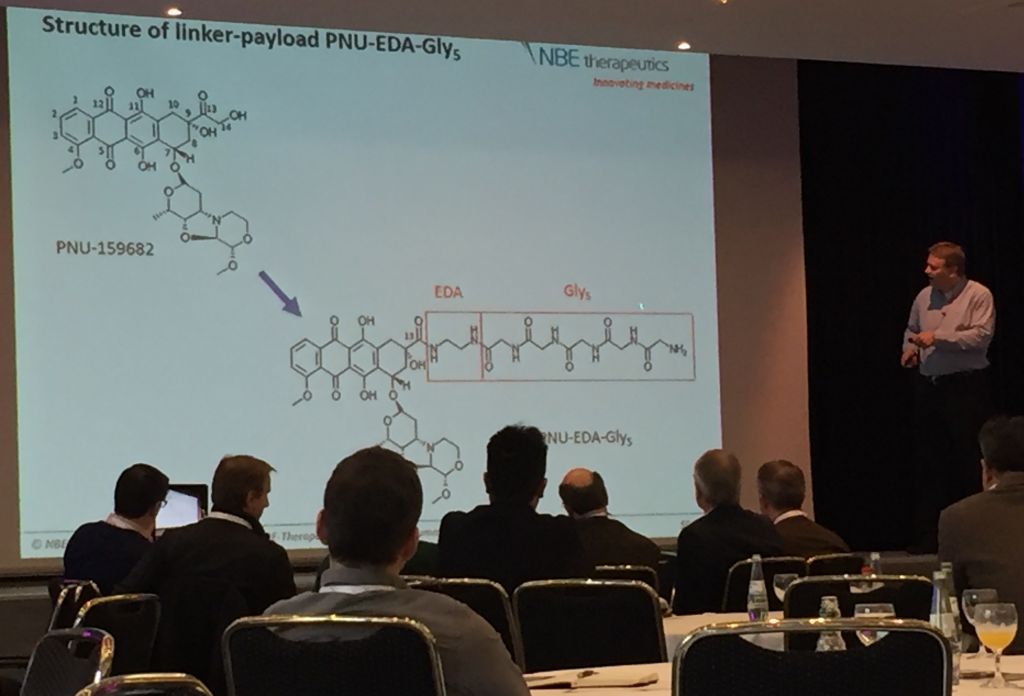
Another linker-related problem in ADC development is the drug-antibody ratio (DAR): it’s difficult to pin an exact number of toxins to an antibody and even more difficult to characterise the consequent distribution. The sortase-mediated antibody conjugation (SMAC) technology that NBE is developing offers itself as a solution to this as well by leveraging the specificity of the enzyme.
Many other talks focused on improving linkers. Robert Kolakowski, a Senior Scientist in Chemistry at Seattle Genetics, dedicated his talks to finding linkers that would accommodate toxins with specific chemistries, and Christoph Rader, an Associate Professor of Cancer Biology at the Scripps Research Institute described the efforts of his group, in collaboration with unnatural amino acid giant, Pete Schulz, to improve site-specific conjugation with selenocysteines.
Meanwhile, an entire company has grown out of research into linker optimisation. Synaffix, based in Oss, the Netherlands, is building a platform of glycosylation site-based linkers. “If you have the aim of developing an ADC, we want to be your partner in conjugation technology,” said CSO Florian van Delft. The company sees these sites as superior to their cysteine counterparts for their improved specificity and tolerability.
While the linker might seem like the least attractive part of an ADC, it is arguably the most important as research efforts into these components continues to expand. Despite the great strides of the presenting companies at the World ADC Conference, much work remains to be done, as the four patient deaths in a trial by Seattle Genetics last December soberly reminds us.
“We’ve had a lot of ADCs go into the clinic, but not many have worked,” said Kurt Gish, an Associate Director of Biologics Technologies at AbbVie. “We’ve chosen some of the best targets — those that are higher in cancer and lower in normal cells…We have some of the best payloads that chemists — some of the most potent substances on earth, that chemists have made even more potent.” If they can be effectively linked to their antibody guides, the dreams of ADC researchers may come true!
Images from the author, NBE Therapeutics and Kateryna Kon /shutterstock.com
Oncology R&D trends and breakthrough innovations