Newsletter Signup - Under Article / In Page
"*" indicates required fields
Update (18/09/2018): Horama has increased the total raised in its Series B fundraising to €22.5M, with the Belgian fund V-Bio Ventures joining the team of investors backing the company. The funds will be mainly directed at developing Horama’s gene therapy for retinitis pigmentosa.
Published on 08/10/2017
Horama has raised a second fundraising round that will support its lead candidate, a gene therapy for retinitis pigmentosa, through clinical trials.
The French company Horama has announced the closing of its Series B round, which has ammassed a total of €19M. Backing the round are four new investors Kurma Partners, Fund+, Pontifax and Idinvest, as well as existing investors Omnes Capital, GO Capital and Sham Innovation Santé.
The funds will be used to finance the development of Horama’s lead candidate, HORA-PDE6B, which is about to enter clinical trials. Last month, the French ANSM authorized the start of a study at the Nantes University Hospital that will recruit 12 patients to test the gene therapy in humans for the first time.
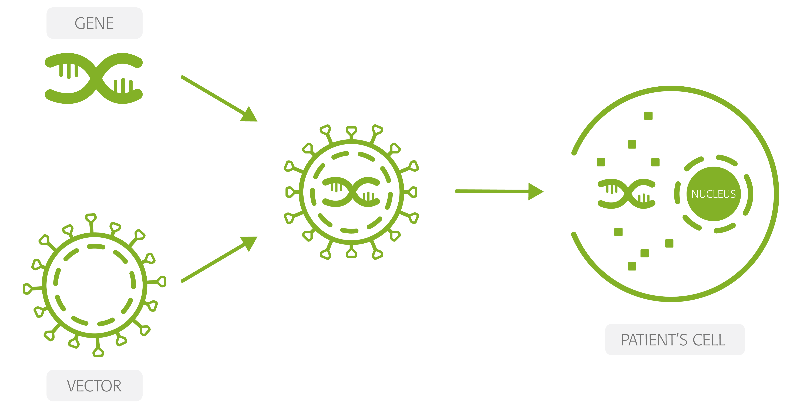
HORA-PDE6B is intended to cure retinitis pigmentosa in patients that develop the disease because of a mutation in the PDE6β gene, which amounts to around 5,000 people in the US and Europe. The gene therapy consists of a recombinant adeno-associated virus (AAV) in which the viral DNA is replaced by a functional copy of the PDE6β gene.
This DNA is injected under the retina, right between the photoreceptors and the retinal pigment epithelium, where the cells use it to express the correct version of the PDE6β protein. And since these cells are not renewed over time, the therapy has the potential to prove effective for years after a single injection.
With the first gene therapy for an eye disease about to enter the market, Horama could be one of the first to bring forth one that addresses retinitis pigmentosa, along with NightstaRx, a British company that is already testing in the clinic a gene therapy for patients whose retinitis pigmentosa is caused by a mutation in the RPGR gene.
Images via AnnaVel / Shutterstock; Horama
Are you interested in eye disease R&D?







