Kiadis Pharma starts Phase III trials with ATIR101, a therapy that could make half-matched donors suitable for bone marrow transplant to treat blood cancer.
From its Amsterdam headquarters, Kiadis Pharma is developing a technology that could solve one of the biggest problems of bone marrow transplantation: finding a donor match. Currently, a bone marrow transplant is the only curative option for patients with blood cancer. But finding a match is difficult, and even when there’s a match, there are often complications such as graft-versus-host-disease, when the transplanted immune system starts rejecting the body it’s been transplanted to.
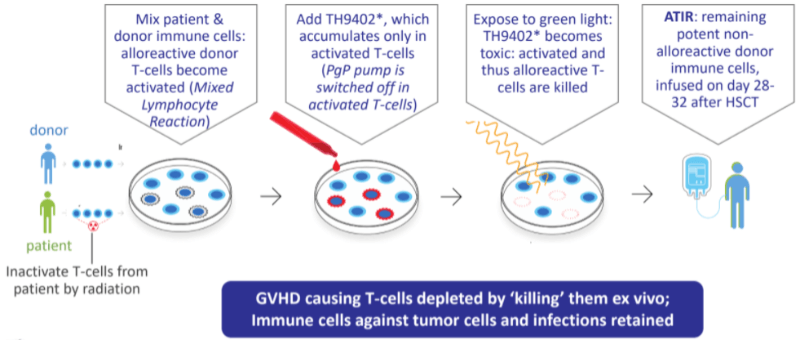
Kiadis’ therapy ATIR101 is designed to remove these complications by removing those cells responsible for rejection and graft-versus-host disease (GVHD) from the mix. This would allow half-matched donors from the patient’s family to safely donate bone marrow without the need for high-dose chemo afterward — a procedure known as the Baltimore protocol.
With the enrollment of the first patient today, ATIR101 has now officially started a Phase III clinical trial that will test whether this approach is effective at improving the safety and efficacy of bone marrow transplants to treat three types of blood cancer: acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL) and myelodysplastic syndrome (MDS).
If Kiadis Pharma can demonstrate that its approach is better at avoiding GVHD and preventing relapse than conventional bone marrow transplant, the treatment could become available as soon as 2019, subject to receiving EMA approval in late 2018.
Kiadis is not the only one developing this approach of eliminating dangerous T cells from marrow transplant. Bellicum Pharmaceuticals in the US and MolMed in Italy are working on their own therapies. But, as Kiadis’ CEO Arthur Lahr explained to me in an interview earlier this year, their approach includes genetic engineering of the T cells and might not be able to discern between dangerous T cells responsible for rejection and beneficial T cells essential to keep the patient safe from infections while they recover from the transplant.
Images via toeytoey /Shutterstock; Kiadis Pharma





