Ablynx has gotten positive results for its candidate (ALX-0171) in Phase I/IIa trial for infants infected with a life-threatening virus – respiratory syncytial virus (RSV).
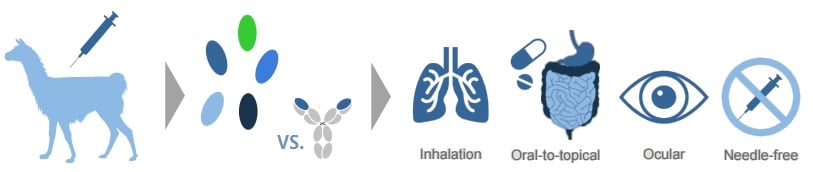
 Located near Ghent (Belgium), Ablynx is making advances in its Nanobody platform, based on the shorter monoclonal antibodies of llamas.
Located near Ghent (Belgium), Ablynx is making advances in its Nanobody platform, based on the shorter monoclonal antibodies of llamas.
Due to their small size, these Nanobodies can be used in a whole new range of pharmaceutical applications – including nebulisation.
This is what Ablynx did for its wholly-owned Nanobody ALX-0171, that is being delivered by inhalation to young children infected with respiratory syncytial virus (RSV).
Now, Ablynx has positive topline results from its Phase I/IIa trial, which enrolled 53 infants aged 1 to 24 months which were hospitalised because of RSV.
The trial met its primary endpoint, which was to show that daily inhalation dose of ALX-0171 is safe and tolerated by infants.
This Nanobody had already shown a good safety profile in adults (in a previous Phase I trial), but these new results are essential for Ablynx to move on to the therapy’s target population – young children.
Early results of the therapy’s effect also look promising. It had an immediate impact in stopping viral replication and reduced the viral load (quantity of virus in the blood).
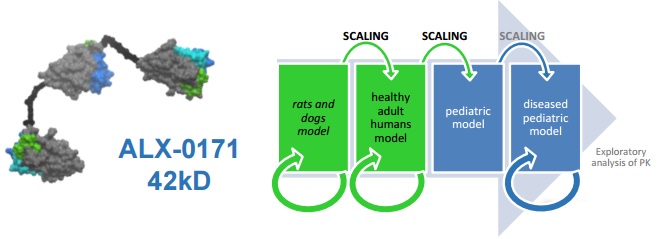
These early signs support the advancement into a Phase II trial, says CMO Robert Zeldin – but this doesn’t seem to be planned yet.
RSV is the primary cause of infant hospitalisation, as well as the virus that causes the most deaths in young children – for which there is no curative treatment available yet.
Besides Ablynx, RSV is also being targeted by several companies like MedImmune (UK), CureVac (Germany), NovaVax (US & Sweden) and ReViral (UK).
These results are an important proof of concept for the inhalation strategy of Ablynx for its Nanobodies, which have already got major scientific validation and Pharma attention.





