Newsletter Signup - Under Article / In Page
"*" indicates required fields
The RAIDs consortium made its closing event last week. After 4.5 years of studies, it has managed to change our appreciation of HPV cervical cancer towards building intelligent targeted therapy designs.
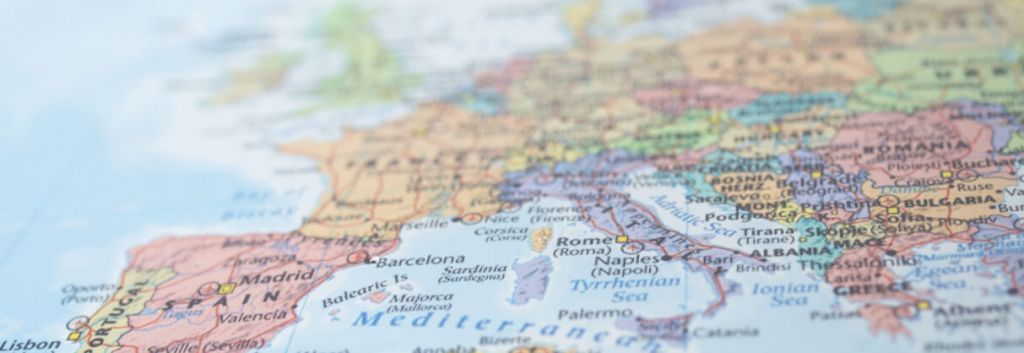
Every year in Europe, 16,000 women die from cervical cancer and another 34,000 are newly diagnosed, which makes it the second most frequent form of female cancer. Of these patients, 90% are infected with the human papillomavirus (HPV), and there is a large discrepancy worldwide with up to 3-4 fold difference rates in incidence and mortality between countries.
Thanks to the improvement in detection and therapies, mortality rates of cervical cancer have decreased by 72% since the early 1970s in the UK. Now, with the personalized medicine revolution, cervical cancer could become a sum of different diseases that can be individually treated, but before getting there the field is still lacking tools. There is a growing need for the development of treatments and biomarkers to follow up the course of the disease.
Stakeholders are answering this call in different ways: companies such as Genticel are developing vaccines, while large consortiums like RAIDs (Rational molecular Assessments and Innovative Drugs selection) are aiming to identify patients pattern and develop drugs based on molecular profiling. It is meant to set the stage for future precision medicine and vaccine development through a comprehensive analysis of the tumor and its interactions with the tumor microenvironment.
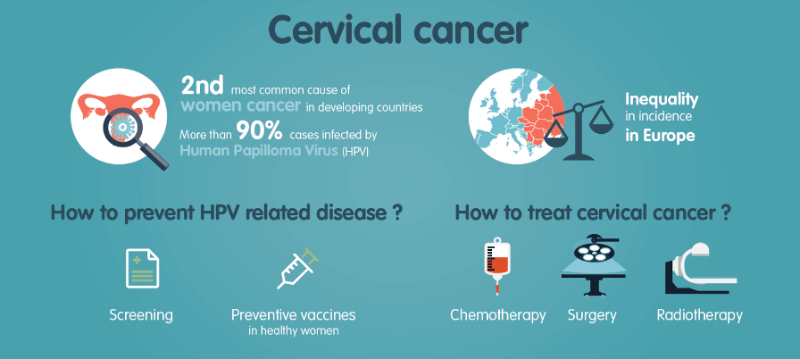
The RAIDs consortium is coordinated by Dr. Suzy Scholl, a medical oncologist at Institut Curie in Paris, and brings together almost 20 public and private partners from Germany, Netherlands, Serbia, Moldavia, Hungary and Romania, the countries that have the highest prevalence of cervical cancer in Europe, doubling that of the UK. Such a promising multi-center trial could only be possible thanks to the authorities that pushed forward the initiative with a European €6M grant.
The study has achieved the molecular characterization of more than 400 patients with proteomic, genomic, blood biomarkers and tumor microenvironment high-throughput analysis. “It is the first time that a big scale study enables the collection of samples integrating clinical and molecular data for cervical cancer,” explains Maud Kamal, Project Manager for RAIDs at the Institut Curie.
The results identified challenges with systematic biobanking, especially regulatory issues. In order to better run international studies in Europe, it is mandatory to review the heterogeneous regulatory rules across the EU, which are currently an obvious brake for multi-country trials. As Dr. Scholl explains, “new adaptations are needed to help creative developments such as platforms involving translational aspects, like biobanking and electronic case report forms (eCRFs), but also clinical trial methodology and supervision in different countries with different cultures and different visions.”
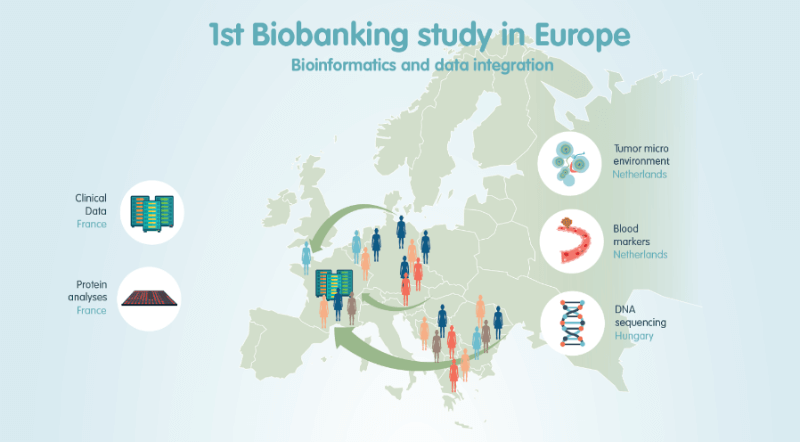
But despite the challenges, the study has led to a patent filing and promising preclinical results regarding the combination of radiotherapy and immunotherapy using a novel therapeutic vaccine for HPV, STxB-E7. Complete tumor remission in mice following the combination therapy was visualized using in vivo imaging techniques. Such targeted therapies could be also being investigated for other HPV-related forms of cancer, but this has to be considered on a case by case basis. “An appropriate algorithm has to be developed for each target, microenvironment and mutational load in order to deliver the right innovative treatment to eligible patients,” explains Dr. Susy Scholl.
BioRAIDS has set the stage for future clinical trials of specifically targeted drugs or drug cocktails. For Suzie Scholl, the next step is to build on the results from the present study in order to identify a series of molecular markers linked to a poor outcome with conventional treatments and specifically target them using innovative therapies. Another milestone will be to develop translational screening platforms that could be partnered with industrial collaborators. Such a database would be very powerful for the biotech industry, enabling retrospective studies on a well-defined dataset regarding drug sensitivity, sophisticated animal modeling or even monitoring genetic changes.
“The RAIDs’ results will be priceless to identify a set of stratification criteria that will help in the near future to better orient patients towards personalized treatments,” confirms Dr. Scholl. In fact, the RAIDs consortium’s future action plan is to identify gene clusters that are associated with poor prognosis and to implement innovative adjuvant treatments in a precision medicine clinical trial called RAIDs2CURE, expected to recruit 800 patients.
The trial is currently under preparation, with organizers looking for funding and partners for this new edition and negotiating with pharmaceutical companies. “Several small companies have been associated with this project with the aim to foster economic progress and we do hope that there will be exploitable results in terms of predictive markers of treatment success as well as new combinations of reagents,” says Dr. Scholl. With such promising results in its first edition, there’s no doubt that the initiative will hook interest from larger pharma.
Images via Zangrilli Andrea / Shutterstock; www.raids-fp7.eu
Oncology R&D trends and breakthrough innovations