Newsletter Signup - Under Article / In Page
"*" indicates required fields
SiSaf Ltd, an RNA delivery and therapeutics company, has announced that SIS-101-ADO, its siRNA therapeutic for patients with autosomal dominant osteopetrosis Type 2 (ADO2), has been granted orphan drug designation by the U.S. FDA.
Also, due to the serious manifestations of this rare skeletal disorder in children, SIS-101-ADO has been granted rare pediatric disease designation for the treatment of autosomal dominant osteopetrosis.
Orphan drug designation provides SiSaf with incentives such as tax credits for clinical trials, exemption from user fees, and expanded marketplace exclusivity. The rare pediatric disease designation entitles SiSaf to apply for a priority review voucher that can be used to have the drug approval process expedited by the FDA.
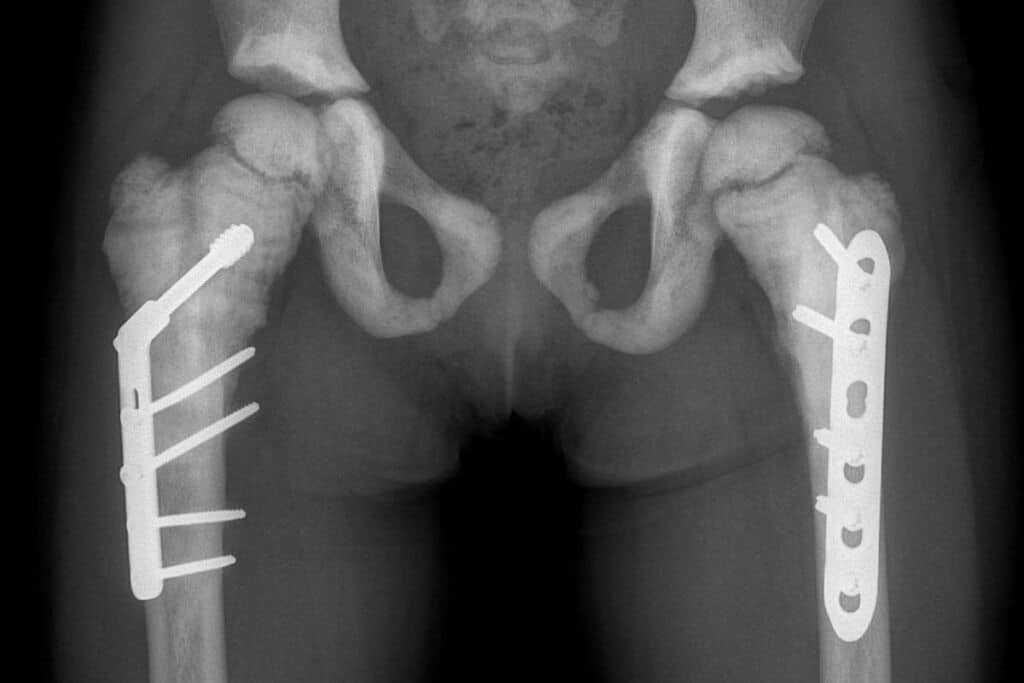
The FDA awarded SIS-101-ADO rare pediatric disease designation on the basis of the serious or life-threatening manifestations of ADO that primarily affect children including blindness from optic nerve compression, anomalies in dental and craniofacial development, and scoliosis.
There are currently no approved treatments for osteopetrosis ADO2 and no other treatments currently in clinical trials. If approved, SiSaf’s SIS-101-ADO would be the first treatment for osteopetrosis ADO2 and could provide life-altering benefits for those who suffer from this debilitating disease. SiSaf is currently preparing for first-in-human clinical trials.
About SiSaf‘ SIS-101-ADO
SIS-101-ADO combines an siRNA that suppresses the expression of CLCN7 with SiSaf’s Bio-Courier delivery technology. By downregulating the expression of CLCN7, a mutant gene expressed by osteoclasts and other cell types responsible for causing ADO2, the RNA therapy restores bone mass and quality to nearly normal levels.
Genetic skeletal disorders such as ADO2 account for 5% of all birth defects globally, yet many unmet needs and challenges remain for providing safe and effective treatments. SiSaf said its Bio-Courier technology has the potential to accelerate the development of new RNA-based treatments. The technology addresses the limitations of other RNA delivery technologies by stabilizing lipid nanoparticles with bioabsorbable silicon.
SiSaf’s founder and CEO, Suzanne Saffie-Siebert, said: “Being granted orphan drug designation and rare pediatric disease designation is a major milestone in our drive to move our revolutionary siRNA treatment forward to alleviate the pain and suffering that osteopetrosis ADO2 inflicts. SIS-101-ADO ushers in the potential for a new era of personalized care and treatment options for ADO2 and other rare bone and skeletal diseases.
“SIS-101-ADO and other Bio-Courier formulated drugs will not only be able to treat rare skeletal disorders but can clear the way for therapeutics for other rare diseases once thought impossible to treat.”
Bio-Courier technology program
SiSaf’s Bio-Courier drug delivery platform builds upon lipid nanoparticle technology with the introduction of silicon, for structural integrity and durability. The result is a nanoparticle with improved RNA loading capacity and protection from hydrolysis, combined with efficient transfection and controlled release of the oligonucleotide payload.
Bio-Courier versatility is achieved by modifying particle size, surface charge and surface ligands for targeting to the desired site of drug action. Bio-Courier formulated drugs can be used for multiple routes of administration.
Earlier this year, SiSaf announced a collaboration with the University of Leipzig in Germany to develop targeted micro interfering RNAs (miRNA) for the treatment of cancer, with an initial focus on pancreatic cancer.