Triastek, Inc. has completed its first in human study of its third 3D printing drug product, T21, designed to treat moderate to severe ulcerative colitis (UC).
Imaging results from the study confirmed that T21 tablets are precisely delivered and released to the target site – the colon – for the drug release.
The administration of oral medication is generally regarded as the preferred method for patients with UC due to its safety, pain avoidance and patient compliance. However, it has been historically difficult to achieve precise delivery of drugs to the colon, without releasing any of the medication in different parts of the gastrointestinal tract.
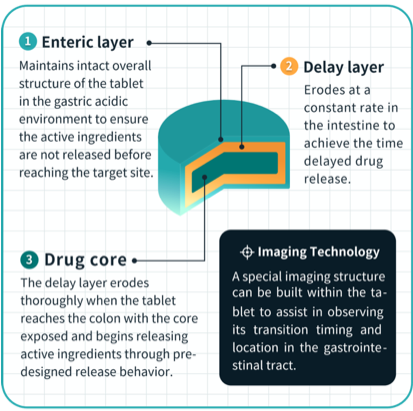
Triastek developed T21 in an effort to solve the problem of targeted and precise delivery of oral drugs to the gastrointestinal tract. Through the company’s Melt Extrusion Deposition (MED) 3D printing process, its 3D printed tablets control when, where and how much medicine is released in the gastrointestinal tract, ensuring a more targeted and efficacious drug delivery.
“The first in human study data with T21 verifies the precise colon delivery capability of the MED process, and this platform is poised to become the novel drug delivery system of choice for colon targeted new product with either local efficacy or systemic absorption,” said Xiaoling Li, co-founder and chief scientific officer of Triastek.
“We hope to continue showcasing how Triastek’s 3D printing processes can bring technical solutions to pharmaceutical companies for efficient product development of optimized drug delivery, ultimately leading to the ability to provide patients with more clinically valuable medicines.”
Triastek aims to develop partnerships with pharmaceutical companies to help them differentiate their products through the use of 3D printing of medicines. Triastek has most recently partnered with Boehringer Ingelheim, Eli Lilly and Merck KGaA.
About T21
T21 is Triastek’s third 3D printed drug product, and is an oral, colon-targeted delivery drug for moderate to severe ulcerative colitis (UC). In November, 2022, Triastek received clearance for its Investigational New Drug (IND) application for T21 from the United States Food and Drug Administration (FDA).
The original drug is an oral Janus kinase (JAK) inhibitor called tofacitinib. Following the approval of T21, Triastek launched FIH research in Q1 of 2023 to verify the transport, erosion process and release site of T21 in the human body after oral administration. At present, T21 has completed the first-in-human study, and imaging results have confirmed that T21 tablets are accurately delivered to the target site – the colon.
About Melt Extrusion Deposition (MED)
Melt Extrusion Deposition (MED) 3D printing is an additive manufacturing, end-to-end process that continuously converts powder feedstocks into softened/molten states followed by precise layer-by-layer deposition to produce objects with well-designed geometric structures.
This optimized product design helps control when, where and how much medicine is released in the gastrointestinal tract, ensuring a more targeted and efficacious drug delivery. The MED process can be used to design and develop new chemical entities as well as highly differentiated lifecycle management products for unmet medical and clinical needs.