Newsletter Signup - Under Article / In Page
"*" indicates required fields
UniQure’s gene therapy for Hemophilia B is performing well in clinical trials, but the company faces many challenges with its current financial situation. Can it manage to make it to the market?
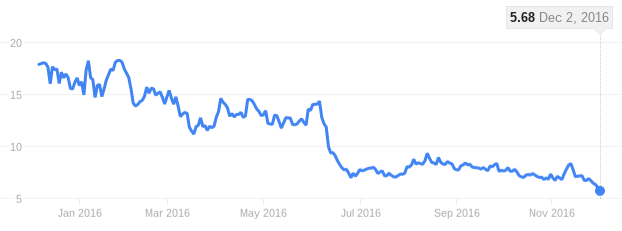
UniQure is famous for launching Glybera, the first globally approved gene therapy, which has been a commercial failure. The company is now indefinitely missing a CEO and facing an all-time low in its stock. But there is still hope for the Dutch biotech, which just announced positive data from its Hemophilia B Phase I/II trial.
Ten patients were treated with uniQure’s AMT-060 gene therapy, which employs an adeno-associated viral vector to deliver a functional copy of the Factor IX (FIX) gene, required for adequate blood clotting. The patients, which have been monitored for up to a year, have shown maintained, dose-responsive levels of FIX activity, with only one report of spontaneous bleeding.
This one-off treatment has the potential to eliminate the need of chronic treatment for the 1 in 30,000 people that suffer from Hemophilia B. However, AMT-060 still needs to prove itself in late phase clinical trials and the company has to keep afloat until then. In an effort to recover, the company is adopting austerity measures and has taken an ax to its pipeline and workforce.
One of uniQure’s main competitors in gene therapy for Hemophilia B, Spark Therapeutics, has also just released positive results from its Phase I/II trial. Nevertheless, its candidate SPK-9001 has induced unwanted immune responses to the viral vector in two out of nine patients.
UniQure seems then to have an advantage in terms of performance; a ray of hope for a company whose future seems to rely on the outcome of AMT-060, its leading program. The Dutch biotech is also supported by a partnership with BMS for cardiovascular disease.
The company may be trying to save itself from financial ruin through deals, but are its products really worth the nearly €1B deal with BMS? Biotechs seem to enjoy boasting about billion-euro partnerships, but STAT News has reported that only an average of 14% of those hyped-up deals are actually paid since most of the money is subject to meeting milestones.
With its last milestone payment from BMS in August 2015, uniQure has only received €61M ($65M) so far. The company will have to prove that its technology is worth it and find a suitable pricing strategy in order to avoid making the same mistakes as with Glybera if it wants to reverse its current negative earnings.
UPDATE (Original publication: 05/12/2016): uniQure has finally appointed a CEO, who will be the 3rd in the span of one year. Matt Kapusta, previously uniQure’s CFO and interim CEO for the last 3 months after Soland’s resignation in September, is now taking the reins.
Featured image by Pratan ounpitipong/shutterstock.com; figure from Google Finance






