Newsletter Signup - Under Article / In Page
"*" indicates required fields
Data from therapy development, especially those involving cells, can quickly mushroom into an unmanageable pile in the lab — what happens when you move to the clinic? We sat down with Venn Life Sciences to discuss effective strategies in data management that might benefit companies looking to bring a therapy like Tregs to market.
 Cell therapies are one of the major trends in biotech, and they’re being investigated as a therapy for everything from cancer to chronic inflammation. Say you’re a company looking to leverage T-cells, the ‘soldiers of the immune system’, to develop a cure for an autoimmune disease or chronic inflammation.
Cell therapies are one of the major trends in biotech, and they’re being investigated as a therapy for everything from cancer to chronic inflammation. Say you’re a company looking to leverage T-cells, the ‘soldiers of the immune system’, to develop a cure for an autoimmune disease or chronic inflammation.
If all goes well, you might succeed in finding a cure in the lab, but what happens when you scale up for clinical trials? From their PhD work, scientists are trained to manage laboratory-scale datasets, but the industrial expansion could pose a daunting challenge, especially when it comes to cell therapies.
As an example, Christian Le Bras, Head of Venn Life Science’s Interactive Response Technology department, walked us through the process of developing a therapy from Tregs, regulatory T-cells that modulate an immune response, and the management of the accompanying clinical data.
Source Material Preparation
To start, each patient’s own blood cells must be collected, processed and prepared. In the lab, you might deal with one or a few; but in the clinic, you’d have to manage thousands. Once blood samples are collected, they have to be purified to obtain the Tregs. These are then “educated” by ex-vivo stimulation with antigens so that they are able to recognise them when they are re-introduced to the body. These choice Tregs are then isolated and scaled up for industrial expansion.
Mass production is where the data gets messy in a hurry: to effectively run clinical trials, enough Tregs have to be produced to cover multiple doses administered over several years by IV injections. To whom do you give which dose and when? Is the material even good anymore? An effective data management system, like Venn’s Interactive Response Technology (IRT), can keep all of this organized for you.
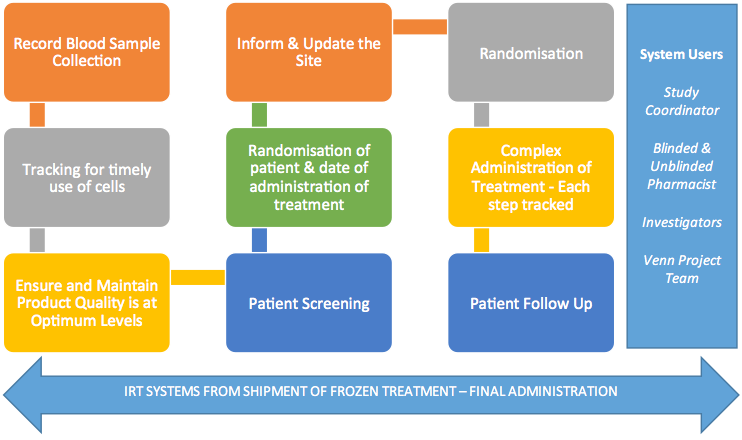
Product Availability
With all of your doses squared away, you also have to make sure they don’t degrade. Cells are fragile and have to be kept under tightly controlled conditions. With this in mind, Venn Life Sciences developed a technology to record and track blood samples as well as target cell density, so a clinician can keep tabs on available treatments.
Once you’re sure of the quality of your samples, you can start treating patients. In Treg therapies, the cells will use the antigens for which they were tuned as a beacon to home in on the inflammation afflicting the patients. The antigens then activate the cells to proliferate and release immune suppressive factors to quell the inflammatory response. Once it has been treated, the Tregs are rapidly cleared.
Sounds like a simple step, right? Perhaps, but the process becomes dramatically more complex when you add the necessary double blinding methodology to guard against bias! Venn’s IRT makes this easier with its random assignment function: the software implements complex stratification to match patients to treatment arms with minimal bias and the option of subpopulation analysis.
Keeping Track of It All
The sheer bulk of this data might seem cumbersome, but it doesn’t have to be. Venn designed IRT to be portable so a clinical researcher can access the database on any web-enabled device and engage with it in real time, whether it’s simply to check numbers, update results or randomize the treatment group of a patient. In fact, Christian says the company’s goal is to make it increasingly automated to require less effort in the management of patient use and drug logistics.
While this technology might sound like icing on the cake, Christian warned us not to underestimate its importance. Even if you have stunning results, they won’t be useful if you can’t find them or spend an inordinate amount of time searching for them. Time is of the essence in bringing a competitive therapy through the clinic and to market: saving a few months can translate to millions of euros in extra revenue. With such tools, we do have the capability of organizing adaptive design that could accelerate the obtainment of positive results.
With this in mind, Venn Life Sciences aims to expedite clinical development by addressing the challenges of tracking samples and results on the huge scale of such trials. Accordingly, the company has designed IRT to provide a streamlined and quality-driven method to manage your data in a more time and cost-effective way.
Want to know more about IRT? Give them a call on +33 1 402 11 970, or drop Venn Life Sciences a line!






