Celyad has received approval to start Phase Ib trials in Belgium with its autologous CAR-T therapy, which can target 80% of tumors.
After a successful Phase I in leukemia and myeloma, Celyad is ready to start a Phase Ib trial for its autologous NKR-2 in 7 cancer indications, now including solid tumors. The new trial, aimed to examine different dosing and treatment periods, has received approval in Belgium and is waiting for FDA clearance to expand to the US as well.
The promises of CAR-T therapy have generated hype in the immuno-oncology field. However, therapies in the clinical stage are facing challenges regarding toxicity and relapse. Celyad is trying to overcome them with a unique technology to express Natural Killer Receptors (NKR) on T cells. The advantage is that they can bind to 8 cancer ligands that not only target cells but also the tumor microenvironment. With this strategy, the company can target 80% of tumors with a single therapy.
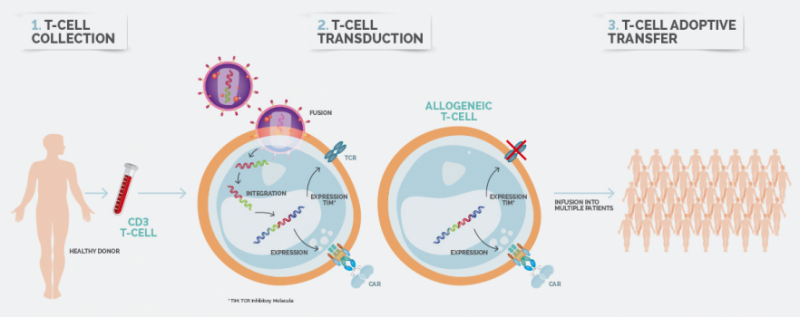
In addition, Celyad leveraged the NKR-T cell platform to build the first only allogeneic CAR-T therapy, which recently received a US patent, and it is expected to hit the clinic next year. By harvesting T cells from a healthy donor, Celyad’s therapy can be manufactured as an ‘off-the-shelf’ product. If the therapy is successful, it could bench all competing autologous CAR-T therapies, which rely on a lengthy process of harvesting the patient’s own cells to be engineered and infused back.
However, the competition is fierce, as shown in the recent public dispute with André Choulika, CEO of Cellectis, about the validity of Celyad’s patent. For its part, Cellectis is implementing a switch control system to activate CAR-T cells only when necessary and reduce toxicity. Its lead candidate, UCART19, recently started Phase I trials. Although both companies are behind Novartis and Kite Pharma, racing to attain FDA approval for their CAR-T therapies, their novel technology could outperform competitors in safety and efficacy.
After a failed therapy for cardiac disease, will Celyad’s unique immuno-oncology therapy make it to the market? The three deaths in Juno Therapeutics’ Phase II proved that CAR-T field is very unpredictable. However, with a product targeting 80% of cancers, Celyad could be swimming in cash by targeting a €100B market.
Featured image by Juan Gaertner/shutterstock.com; figure from Celyad.