Diagnostics have been thrown into the spotlight in the Covid-19 pandemic because of their instrumental role in preventing further infections. However, developing a test for Covid-19 isn’t an easy task. What exactly is needed to make a good Covid-19 test, and how are European biotech companies working to fill this need?
While the development of vaccines and other treatments for Covid-19 is getting the bulk of the media attention, the role of diagnostics is also coming to the fore.
Testing is crucial to detect infected individuals, particularly healthcare professionals, so that they can be treated and quarantined to avoid spreading the disease. The information provided by the tests can also allow world leaders to devise plans for controlling the spread of the virus, and to develop successful strategies for ending social distancing or isolation policies. A good example of testing in action is South Korea, which was able to control the virus better than many other countries because of the country’s widespread testing and social contact tracking.
What makes a useful test for Covid-19?
With a call to “test, test, test” in March, the World Health Organization emphatically pushed the importance of testing for Covid-19. This call has been met with an unprecedented explosion of diagnostic products in development around the world to detect the disease. While there is huge variation in the types of tests available, there are some key expectations that they must meet to be useful in this pandemic.
First of all, the accuracy of a test is essential. A good test should be able to detect positive cases even when the levels of the virus (or the immune response to it) are extremely low, while not generating false-positive results. Additionally, to get European approval for clinical detection of Covid-19, diagnostics companies need to prove that future manufacturing batches of the test will be as accurate as the first.
According to Mikael Kubista, CEO of the Swedish diagnostics company Tataa Biocenter, another vital characteristic of a good Covid-19 test is robustness. He told me that Covid-19 testing protocols must be standardized and reproducible at all stages of the testing process, including sampling and transport.
Given the enormous scale of the current Covid-19 pandemic, tests need to be usable in bulk. Vicente Pelechano, a researcher developing a Covid-19 assay at the Swedish Karolinska Institutet, explained that factors such as the availability of the test’s components, affordable infrastructure, throughput, and ease of use are all crucial for achieving this.
“If you have the more sensitive method but cannot afford it, or it can only test 1% of what you need, that is not the right test for that context,” Pelechano added.
There are some non-essential characteristics that can still be useful in Covid-19 testing. For example, the ease of sampling, the time taken to get results, and the type of diagnosis the test provides can all prove powerful tools for combating the pandemic.
Testing for viral RNA from throat swabs
The majority of the available tests for Covid-19 belong to a group that detects the virus’s genetic material, RNA, in patient samples. They usually start with taking a swab from the back of the patient’s throat and sending it to an official diagnostics lab. Once in the lab, a technique called reverse transcription polymerase chain reaction (RT-PCR) can then show whether the genetic material from the virus is present within a few hours.
Suppliers worldwide are selling RT-PCR tests to European countries. In Europe, some of the leading suppliers of these tests include the German company Qiagen and Swiss giant Roche. Other European companies also gunning to get a share in the Covid-19 RT-PCR test kit market include the French diagnostics supplier Novacyt, whose test was launched in February, and Eurofins, a company in Luxembourg.
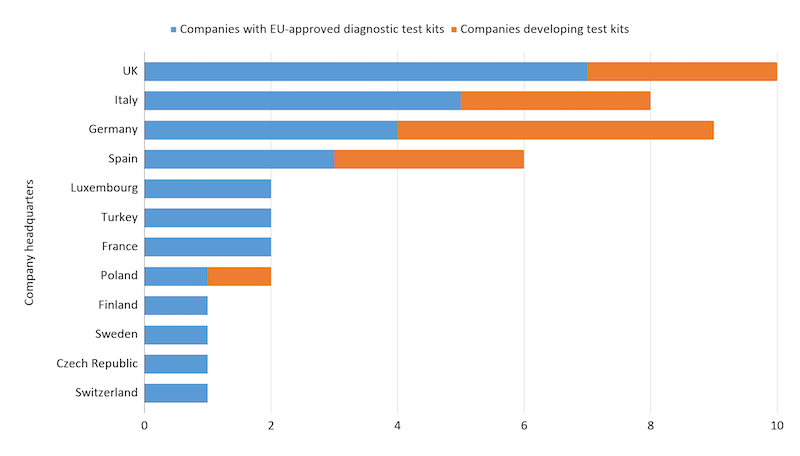
While there are companies developing RT-PCR tests in a large number of European countries, the UK, Germany, and Italy seem to host the most activity in this space.
Companies developing RT-PCR testing kits for Covid-19 in Europe as of 10/06/2020. Data from public press releases.
Theoretically, RT-PCR tests have excellent accuracy, provided that the sample is collected and processed correctly. However, some tests may be more likely to give false-negative results for mild cases or when good quality samples are not available. Additionally, the results might take days to come back because samples must be sent to lab facilities.
This speed issue is the focus of intense innovation at present. For example, Molecular Biology Systems in the Netherlands is developing a machine that detects Covid-19 presence in just eight minutes. As another example, BforCure in France recently received a €1.8M grant to advance its ultra-fast RT-PCR technology, which promises to give results in less than 30 minutes.
One type of innovation called RT-LAMP could make RT-PCR testing more suitable for testing on location. RT-LAMP tests produce a ‘yes’ or ‘no’ type color change in a liquid if the viral RNA is present. This technique can detect Covid-19 quickly and without large capital equipment. The technology is currently being used in an EU-funded research project seeking to develop fast Covid-19 tests.
For Pelechano, whose group is working on a Covid-19 test based on RT-LAMP, the biggest challenge was the speed required to develop a test during a crisis. “We learn more every week,” he said. “In academic science, we are used to working at a slower pace with time for reflection and improvement.“
Although RT-PCR is still the dominant technique for diagnostics, scaling up testing has been patchy across different nations in the latest pandemic. One of the reasons for this is the issue of limited supply. For example, suppliers such as Copan Diagnostics in Italy have been in the news as one of the world’s few suppliers of nasopharyngeal sampling swabs. Another example is the case of supply delays from the German supplier Qiagen after the US Centers for Disease Control included their kits in a protocol.
Assessing the blood
While serological tests have long existed for viruses such as HIV, their use in Covid-19 is not as advanced in Europe as that of RNA tests. The aim of these tests isn’t to screen for the virus itself, but to gauge whether the patient’s immune system has reacted to the virus.
Many easy-to-use serological tests have been widely cited in the media recently as companies announce availability and regulatory approval for Covid-19 testing. The Irish company Assay Genie, for example, announced the availability of such tests in March.
The most user-friendly versions function in a plastic cartridge, similar to a pregnancy test. They just require a couple of drops of blood to one end of the cartridge. In these so-called ‘lateral flow’ tests, the blood sample mixes with synthetic Covid-19 viral proteins in the cartridge. If the patient’s blood contains antibodies against the viral proteins, there will be a visible signal within minutes.
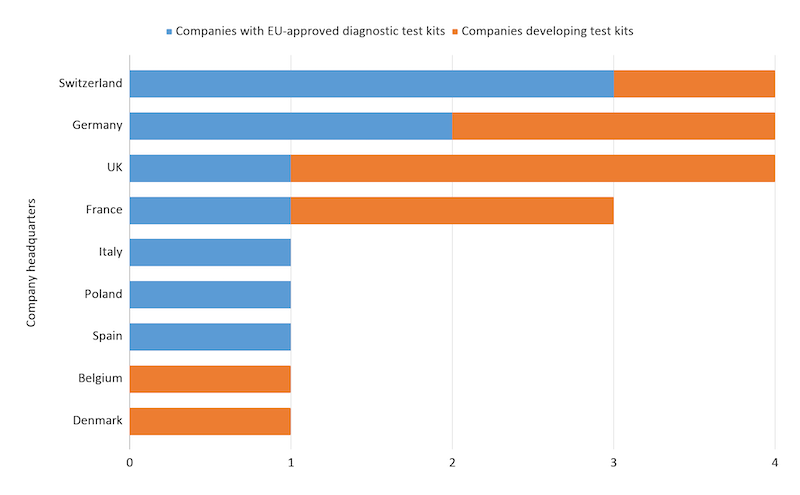
Leading European companies developing serological tests for Covid-19 include the Swiss firms Roche and Quotient, with other developers including the Italian Diasorin and UK Edinburgh Genetics Limited. So far, Switzerland, the UK, and Germany seem to have the most players in this space.
Companies developing serological testing kits for Covid-19 as of 10/06/2020. Data from public press releases.
One big strength of serological tests is that they can be faster and easier to use for non-experts than RT-PCR testing. Another advantage is that, with additional analysis, it is possible to tell whether the patient’s immune system has recently responded to the virus or whether it has had time to develop a more mature, potentially long-lasting defense.
The biggest issue with rolling out these cartridge-based tests in Europe at the start was their accuracy. For example, Spain and the UK bought millions of serological tests from suppliers in Asia — a manufacturing hub which had a head start in testing — only to discover that many of the tests did not perform well enough. To add to the evidence, a group at the University of Oxford screened many commercial serological tests and found that they do not perform well compared to the ‘gold standard’ laboratory technique for serological testing, called ELISA.
Similar to the case of RT-PCR tests, there has been an explosion of product development launches for serological tests for Covid-19 in the hopes of making an accurate, affordable test available in bulk. However, it’s unclear whether these will become widely available and in sufficient quantities to be able to influence policy decisions or personal behavior during the first wave of the Covid-19 pandemic, which could wind down in the coming months.

Emerging types of Covid-19 testing
In addition to the traditional testing approaches, funding agencies and companies have been investing significantly in novel types of tests for Covid-19 that could allow easier sampling, automation, and new types of information.
One key example is the US Sherlock Biosciences’ Covid-19 test that employs the gene editing tool CRISPR/Cas9 and was given an emergency use authorization by the FDA earlier this year. Another less advanced case is the company Photonics21 in Spain, which develops photonics tests that use ultrasensitive lasers to detect low quantities of virus in saliva samples as early as the first day of infection.
Other emerging innovations include testing for viruses in the breath of patients and Covid-19 detection in wastewater in order to monitor its spread across the whole population.
In more of a research capacity, a number of genomics companies like the Austrian firm Ares Genetics are tracking the evolution of the coronavirus responsible for Covid-19. Understanding the way the virus evolves could be valuable for detecting the emergence of new and more virulent strains of the Covid-19 virus, giving policymakers precious time to react to the new threat.
How will Covid-19 affect diagnostics innovation going forward?
There has been an unprecedented global economic downturn caused by social distancing initiatives, travel restrictions, and, in parallel, a refocusing of efforts by healthcare companies to develop and manufacture diagnostics for Covid-19.
The EU made it harder for diagnostics companies to market tests in 2017 by tightening regulations. With the pandemic now raging, though, the EU has accelerated the uptake of new diagnostic tests onto the market with ‘emergency use authorizations.’ According to Kubista, these authorizations “make the route to market affordable for smaller innovative companies, hopefully leading to much-needed competition.“
Both Kubista and Pelechano felt that Covid-19 testing has been insufficient in Europe and that it is, of course, easier to say now retrospectively what should have been done from a policy standpoint. For example, adjustments could have been made in the transport of viral samples to cut costs.
Even after the pandemic, one can imagine that there will be a continued need for Covid-19 diagnostic testing in hospitals and that more routine testing, including rapid testing on location, will be performed.
“There will be a stable low level of positive cases leading to travel restrictions and countries requiring border testing,” Kubista said. “Patients entering emergency care units, doctors’ offices, visitors to retirement homes… are all examples where point-of-care diagnostics will be important.” Pelechano, meanwhile, sees a future with automated testers for multiple diseases in airports and communication hubs.
Companies involved in testing for Covid-19 should have started capturing their share of this testing market — or have made heavy investments to do so — by now. They should also have started planning for life after Covid-19. A general rule in product marketing is that once it is clear what the market need is, it’s generally too late to create a commercial offering.
Both Kubista and Pelechano believe that increased public awareness and investment in diagnostics will last. “Having the whole world in this situation will push the diagnostic field a few years ahead,” added Pelechano.
It’s now down to diagnostic companies to prove that they have what it takes to weather these changes and thrive in the new post-Covid-19 age.
Images from E. Resko, Shutterstock, and Mark Livingstone





