Newsletter Signup - Under Article / In Page
"*" indicates required fields
Biotech is constantly changing, which means that new key terms are popping up all the time. We’ve put together The Labiotech Dictionary of Important Biotech Terms to bring you up to speed.
New and exciting findings come thick and fast in the biotech industry, making it hard to keep up with this ever-changing field. Whether it’s the approval of a new cancer therapy or a potential HIV cure progressing well through the clinic, it’s best to be prepared so that you’re ready for the potentially life-changing findings being made.
Here at Labiotech, we believe that it is important for everyone to follow the changes in the field that could help to save or improve their lives. For that reason, we wanted to break down some of biotech’s key areas, making it easier for everyone to get involved – no matter their background!
Here are some important areas of biotech that we think you should know about.
At Labiotech, we like to give a bit of background about the company that we are covering, as well as the drug, therapy or device that it is developing. These are the key terms that we use to give you an idea of its size and stage of development.
Biotechs vs Biopharmas
Companies in both industries work to produce new drugs for the treatment of various diseases but they take slightly different approaches. Biopharmaceuticals and pharmaceuticals companies tend to be large, using biological or chemical technologies to develop drugs, respectively.
Biotechs are small and may have spun out from a larger company or research institution. As with biopharma companies, they take advantage of living organisms or their products to treat disease. Biotechs focus on research and development (R&D), not enjoying much return on their investment until a drug is found, which might attract investors.
They also focus on a wider range of applications than biopharma and pharma companies, including the development of animal- or environmentally friendly food and bioplastics to reduce the strain on the environment.

However, it is worth keeping an eye on this situation. Biopharma and pharma companies are struggling to keep up with highly innovative biotechs, which could force them to begin following the lead of their smaller counterparts by moving into fields like cell and gene therapy.
Startups
Startups are renowned for their young, relaxed atmosphere, but there is a lot more to them than that! A startup is at the very early stages of development and has so far been financed by its founder(s). However, high costs and low revenues mean that, before long, they must search for financial support from investors like venture capitalists.
Venture capital sees investors put money into early-stage firms with high potential to make a profit. The money goes towards a company’s fundraising rounds or its initial public offering (IPO) when it goes from being a private firm to a publicly owned company. A good indicator of an exciting company is if its fundraising rounds are oversubscribed, as many investors jump at the chance to invest with the expectation of making good returns on their investment.
Labiotech itself is a startup that is still evolving quickly. To find out how we got up and running, check out our story so far.


We cover biotech news every day so regularly come across the confusing terms that biotechs use to describe their new drug and its progress through the clinic. We’ve broken them down so that you can handle them with ease.
Treatments and Cures
The aim for many biotechs is to develop new treatments and cures for various diseases. Both come in many forms, whether it’s a drug, cell or gene therapy, or surgery. A treatment is an ongoing process that brings the symptoms of a disease under control, allowing the patient to live as normal a life as possible. In contrast, a cure is the ultimate goal as it leaves the patient no longer requiring treatment with the condition resolved.
Endpoints
Endpoints are used in clinical trials to measure exactly how safe and effective a new drug is to help decide whether or not it should be approved. These may measure the survival rate of patients that use the drug, record the frequency of side effects, or identify the best dose to use. At each stage of the clinical trial process, new endpoints will be set that the new treatment must meet before being allowed onto the market.
Overall vs Progression-Free Survival
These are two popular endpoints used during clinical trials, particularly those investigating new cancer drugs. Overall survival measures the percentage of study participants that survive for a set period of time after diagnosis or the initiation of treatment, and boosting this is the best way to prove that a drug is effective. Progression-free survival tracks how long during and after treatment a patient’s disease doesn’t get worse, and it is the next best endpoint for a drug to meet. Even better, why not tick them both off at the same time, which Erytech managed for the treatment of pancreatic cancer.

Small Molecules
Small molecules are tiny, chemically manufactured and make up over 90% of the drugs that are currently available on the market today, including aspirin and ibuprofen. They are processed into tablets or capsules, which dissolve in the gastrointestinal tract and pass into the bloodstream where they can reach almost anywhere in the body. Their small size also helps them to easily cross the cell’s membrane.
Antibodies
Antibodies are big, Y-shaped proteins that are produced by B cells of the immune system that recognize antigens – ‘foreign’ proteins found on the surface of disease-causing organisms or toxins. Antibodies detect then bind an antigen via their antigen-binding site to neutralize toxins produced by the pathogen or flag it up to the immune system for destruction.
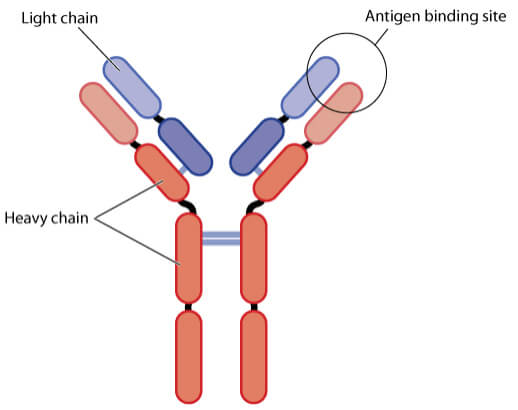
Some of Europe’s best biotechs like Cambridge Antibody Technology and Abcam have been working in this space. MorphoSys is another leading biotech focusing on antibodies, which had its first product approved by the FDA earlier this year and the company is now looking to take on cancer.
Cell Therapy
Cell therapy uses living cells to fight disease or repair damage. These cells may come from the patient, an autologous source, or from a donor, making them allogeneic cells.
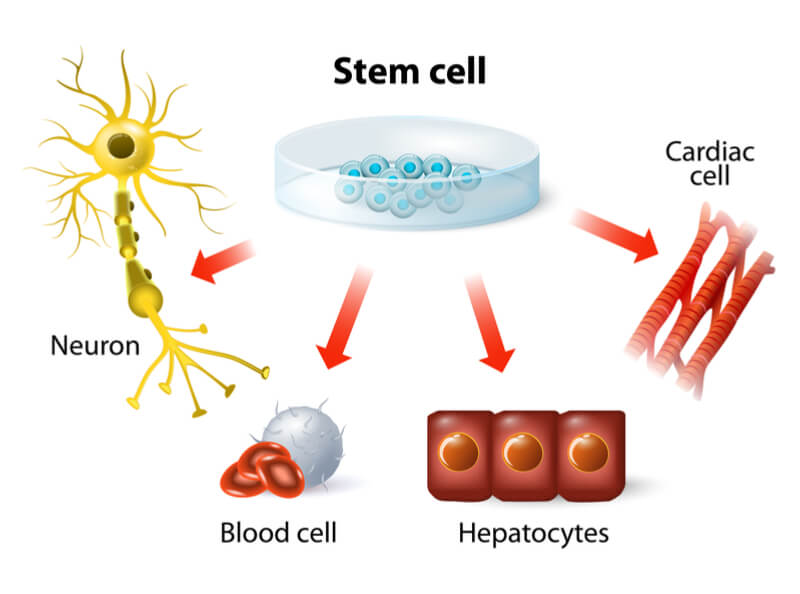
Regenerative medicine uses stem cells, which can develop into many cell types in the body, to replace those lost or damaged by injury or disease. They may be taken from the bone marrow for use in hematopoietic stem cell transplants, or manipulated in a dish to produce a specific cell type.
In addition, we can now reprogram cells in our body like skin cells to produce induced pluripotent stem cells (iPSCs), which have some of the characteristics of stem cells but fewer ethical concerns.
Gene Therapy
Gene therapy alters the expression of a particular gene that may be producing a faulty copy of an important protein, or simply not producing enough of it. Pieces of DNA or RNA are delivered into cells using viral vectors, making the most of their natural capacity to infect cells, or artificially using techniques like electroporation to disrupt the cell membrane.
Once the DNA or RNA is in the cell, a faulty protein can be replaced or its expression can be boosted to regular levels so that regular function is restored. This approach could help to cure diseases caused by a mutation in the genome, including hemophilia and cystic fibrosis.

Biotech is working on all sorts of exciting technology that could change the way we fight disease, feed ourselves and fuel our cars. Here are some of the areas we think that you should be watching particularly closely.
CRISPR-Cas9
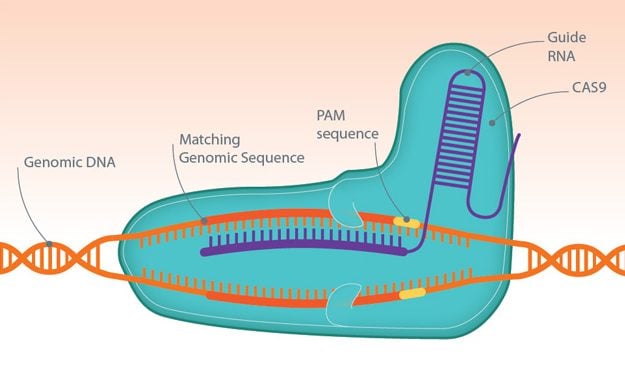
Clustered regularly interspaced short palindromic repeats (CRISPR) is a genome-editing tool that has opened the door to a new generation of drugs and therapeutics. It is used in combination with Cas9 nucleases, enzymes that form part of the bacterial immune system, that can quickly and accurately cut through DNA.
Short pieces of ‘guide RNA’ tell Cas9 where to make cuts in DNA, with different sequences sending the enzyme to various sites in the genome. At the target site, the enzyme will create a double-strand break, which knocks-out the gene of interest so that the effect of this can be investigated. Additionally, a new piece of DNA can be delivered into cells to restore the normal function of a faulty gene or protein that causes disease.
The number of applications for this technology is growing, whether it’s accelerating the production of animal disease models, identifying new drug targets, or treating diseases like the blood disorder, β-thalassemia.
CAR-T Therapy
In the words of Michel Sadelain, Co-Founder of Juno Therapeutics, CAR-T therapy is “at the same time cell therapy, gene therapy, and immunotherapy.”
CAR-T alters cells in our immune system to enhance their ability to fight diseases like cancer. Excitement has grown, with impressive remission rates of up to 94% observed during clinical trials, but this approach is not without its risks, including severe side effects like neurotoxicity and cytokine storm syndrome.
CAR-T therapy sees patients have their T cells – specialized immune cells – extracted, genetically modified in the lab and then infused back into the blood. The engineered T-cells express a Chimeric Antigen Receptor (CAR) on their surface, which, alongside a target-binding domain, allows the recognition of a specific antigen on tumor cells. Once the CAR-T cells detect their target, an internal activation domain triggers the CAR-T cell to bind and destroy cancer cells.
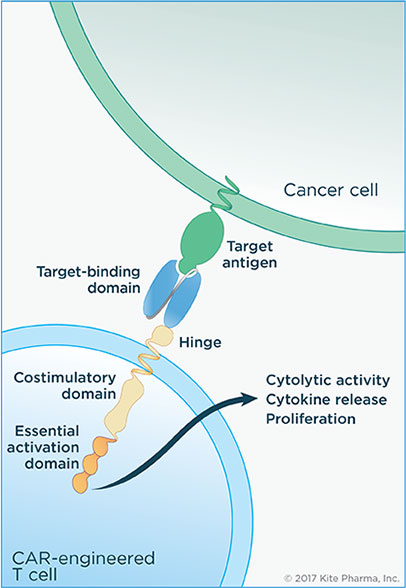
With the first two CAR-T cell therapies receiving FDA approval in 2017 and over 300 clinical trials underway, this is definitely an area to continue watching in 2018.
RNA Therapies
Nucleic acids like RNA were discovered almost 150 years ago, but it is only now that major developments have been made that could see it used therapeutically for the treatment of diseases like cancer, cystic fibrosis, and hemophilia.
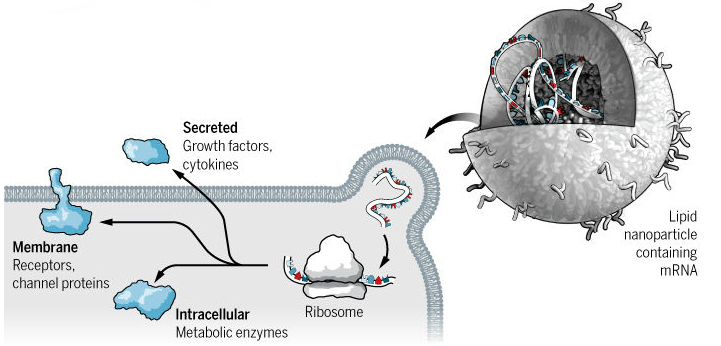
One technique is to use messenger RNA (mRNA), which is a molecule produced according to the cell’s genome that provides the blueprint for protein synthesis. Simply delivery mRNA into a cell allows a protein that is missing or faulty to be replaced. Scientists are slowing overcoming obstacles to its use, including the immune system and RNAse enzymes, which can destroy the nucleic acid before it can have an effect.
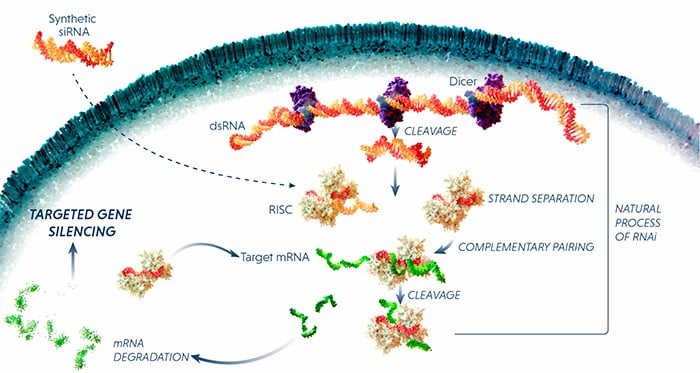
Another approach is RNA interference (RNAi), which uses small pieces of RNA to block the expression of a specific protein. The RNAi molecule binds to mRNA and flags it up for destruction by the cell’s RNA-induced silencing complex (RISC). In fact, the first RNAi drug, patisiran, by Sanofi and Alnylam could be launched in the US and Europe next year.
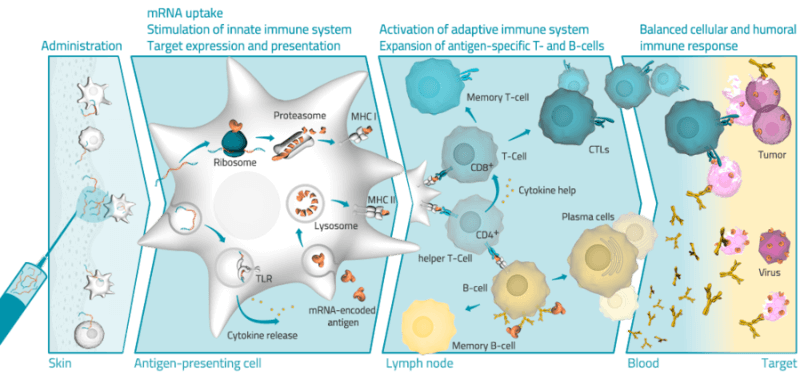
Finally, mRNA could improve vaccines against infectious diseases and cancer. CureVac is hoping to develop vaccines for common colds, hepatitis and HIV. The biotech’s vaccines force the cell to express viral proteins that are recognized by the body’s immune system, which is stimulated to fight the infection. BioNTech has tested its cancer vaccine in 13 patients, which successfully activated the immune system in each of them.
Human Immunodeficiency Virus (HIV)
HIV infects and integrates into the genome of CD4+ T cells – specialized cells of the immune system – impairing their capacity to fight infection. The immune system begins to deteriorate until it can no longer fight infection and disease, which puts the individual at risk of nasty infections and cancers, and developing acquired immunodeficiency syndrome (AIDS).
Current treatment of the disease is antiretroviral therapy, which targets different stages of the virus’ life cycle to slow the progression of the disease. Although these have reduced annual mortality by 45%, the number of new cases and deaths remains high.
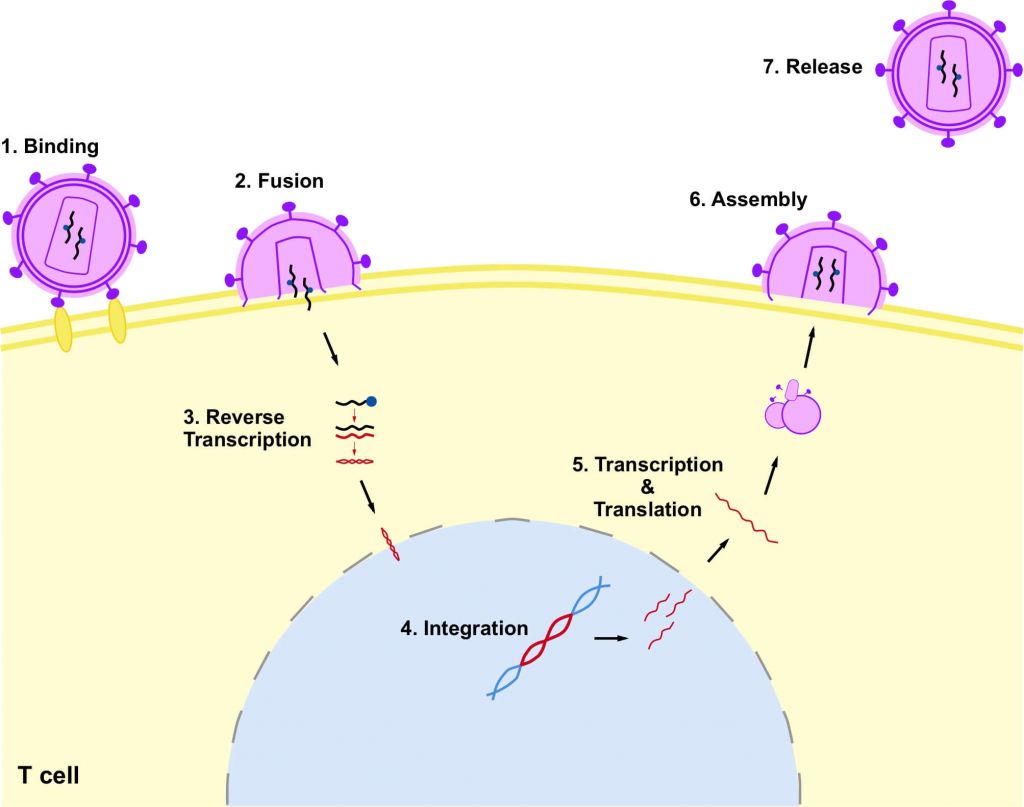
With more than 36 million people affected by HIV/AIDS worldwide, the hunt is on to find a new and effective treatment or even a cure. Abivax is one biotech that is very active in this area, and it recently saw its drug significantly reduce the amount of the virus in patients.

If a drug is to make it onto the market, biotechs need to impress different agencies depending on the where they want to launch it. Here are the big names in drug approval and the process that biotechs must undertake to bring their drug to market.
European Medicines Agency (EMA)
Now that a coin toss has decided that the EMA will move to Amsterdam, it can continue controlling the medicines that make it onto the European market. The agency makes its decisions by looking over the data collected during clinical trials to make sure that new drugs are safe and effective. It is helped by the Committee for Medicinal Products for Human Use (CHMP), which performs the early evaluation of drugs before giving the EMA its opinion.
The agency also boosts the development of new medicines and makes sure that patients in need can access them, ensures that drugs on the market are safe post-approval, and provides clear information about medicines to professionals and patients.
US Food & Drug Administration (FDA)
As the USA’s equivalent of the EMA, the FDA ensures that new foods, drugs, and cosmetics are safe and effective for use. It also regulates the manufacturing, marketing, and distribution of tobacco to reduce its use by minors. If a particular disease is underserved and patients require new medications as soon as possible, the FDA can also speed up the approval of new drugs by awarding Fast-Track or Orphan Drug Designations.
Marketing Authorization Application (MAA)
This is the process that biotech and pharma companies undertake to get their product on the market. In the EU, it is made up of 7 steps:
- Submit an eligibility request to check the company’s product is suitable for consideration.
- Notify the EMA of its intention to apply.
- Appoint rapporteurs to lead the evaluation of the product.
- Organize pre-submission meetings to receive advice.
- Confirm the MAA submission date and submit it to the EMA.
- The CHMP evaluates the application and provides its opinion.
- The European Commission will make its decision within 67 days of receiving the CHMP’s opinion.
By getting to grips with these terms, we believe that you’re ready to dive into biotech and keep up-to-date with all of the exciting research and developments taking place. If there is a word that you think is missing, let us know in the comments below and we will see what we can do.
Images – Photo Melon, ranjith ravindran, Elnur, Zolnierek, Zolnierek, Blamb, Designua, kazu326, unoL, StunningArt, Alessandro Zappalorto, g0d4ather / shutterstock.com, Kite Pharma, V.Altounium / Science, Silence Therapeutics, CureVac, image by author








