Newsletter Signup - Under Article / In Page
"*" indicates required fields
New approaches to tame the immune system in the fight against cancer are getting us closer to a future where cancer becomes a curable disease. Personalized vaccines, cell therapy, gene editing and microbiome treatments are four technologies that will change the way cancer is treated.
Curing cancer is certainly one of the big challenges of the 21st century. Our knowledge of cancer has greatly improved in the last two decades. This has revealed the huge variability that can be found between not only different types of cancer, but also between patients with the same type of cancer.
It seems increasingly evident that there won’t be a single ‘cure’. Rather, each patient will be treated accordingly to their specific needs. But for personalized medicine to become a reality, we need a range of therapies wide enough to cover the whole spectrum of cancer.
In recent years, there has been a surge of new technologies aiming to help the immune system identify and attack tumors, a field known as immuno-oncology. These technologies could make a big difference in the way we treat cancer, taking us closer to being able to ‘cure’ this disease.
Personalizing cancer vaccines
Cancer is caused by genetic mutations that transform healthy cells into tumor cells. These mutations are often at the center of new therapies for cancer; however, they can be very different in each individual tumor.
“Mutations are random. If you look at one patient’s tumor and compare it to another patient’s, it would be highly unlikely that there will be a match,” Sean Marett, CBO and CCO of German immuno-oncology company BioNTech, told me. BioNTech is developing therapeutic vaccines that are created for each individual tumor. “Each patient gets a tailor-made product just for them,” said Marett.
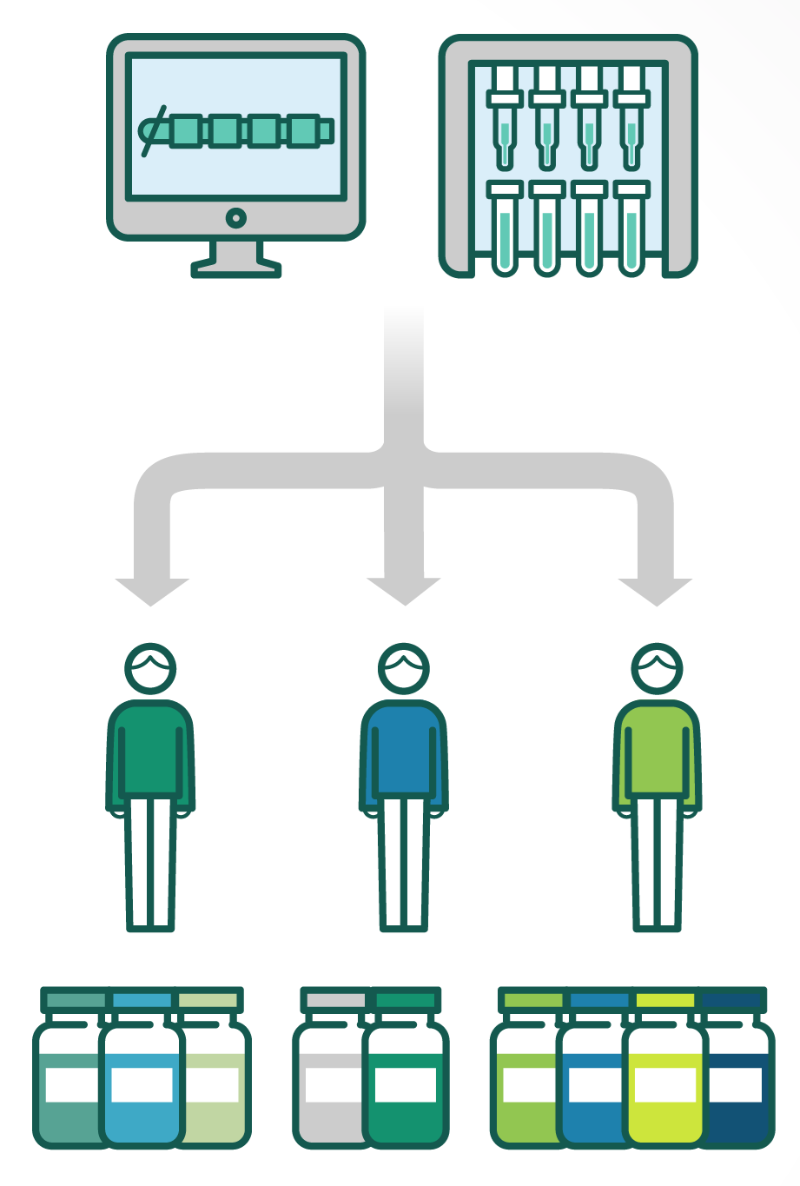
By comparing the DNA sequences of the tumor and of healthy cells, the company can identify multiple cancer mutations and select the ones that are more likely to provoke a strong reaction from the immune system. The vaccines are given in the form of messenger RNA, a molecule that gives cells the instructions to create a particular protein, in this case a cancer antigen that primes the immune system against the tumor.
Unlike with gene editing, the vaccines do not directly edit human DNA, but just provide the message. Another advantage is that the production of messenger RNA is cheaper than that of other new cancer technologies such as cell therapy.
Results from early clinical trials have shown promise for BioNTech’s personalized vaccines, which are being developed in collaboration with Genentech. Marett expects the technology could be ready for the market in the early 2020s.
Still, the company is yet to establish a manufacturing system that can reliably produce a different product every time. “Even if you have the most efficacious product in the world, hospitals will not use an unreliable supply,” pointed out Marett.
He argues that these personalized vaccines could be particularly useful for certain types of cancer that often carry high numbers of mutations, such as lung and bowel cancer. For cancers with lower mutation loads, like ovarian or prostate cancer, other technologies, such as CAR-T therapy, might be more suitable for the patients.
Guiding immune cells to attack
In 2018, we saw the first approval of a cell therapy for cancer. The technology, called CAR-T cell therapy, consists of taking immune T-cells from the patient and genetically engineering them to target a specific cancer antigen.
“CAR-T is changing the treatment paradigm for cancer by creating targeted treatments that are specific to cancer cells,” said Christian Homsy, Executive Director of the board of Celyad, a Belgian CAR-T developer. “Our goal is to develop precise, targeted treatments that eradicate disease while sparing healthy tissue, and in doing so, improving patient lives.”
Indeed, CAR-T clinical trials have shown impressive results in patients that relapse and have exhausted other treatment options. However, the technology has also shown some severe side effects that led to patient deaths.
“With the potency of CAR-T, what you want to absolutely ensure is that the target antigen is not expressed on normal tissue, because if it is you can really wipe out organs and potentially severely handicap or even kill the patient,” said Marett.
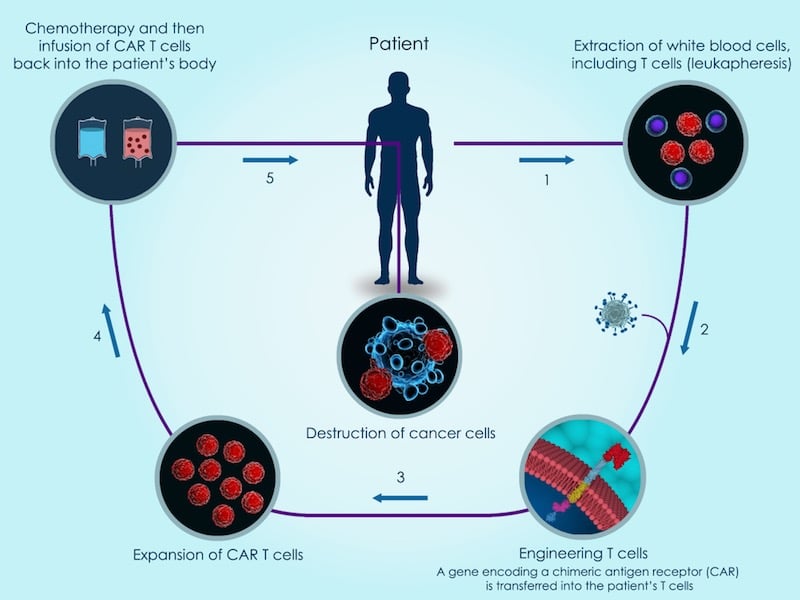
The technology is so far only available for treating certain rare forms of blood cancer. Several players are developing a new generation of CAR-T treatments that can target a wider range of cancers.
Among these players is Celyad. The company engineers T-cells to carry a molecule, borrowed from another type of immune cells called natural killer cells, with the capacity to target 80% of cancer cells. This could make it effective not only against blood cancers, but also in solid tumors, which are a challenge for cell therapies.
“Celyad’s CAR-T approach exploits the molecular differences between cancer and healthy cells, homing in precisely on the cells that cause disease. These targeted approaches will hopefully lead to more tolerable treatments with higher efficacy and cure rates, particularly for patients with limited treatment options,” said Homsy.
Another issue with the technology is its six-figure price. Part of the reason is that the therapy has to be manufactured individually using each patient’s cells. Several companies are developing off-the-shelf versions that can be derived from a donor with a simplified manufacturing process. Celyad and the French company Cellectis, the first to run clinical trials with these off-the-shelf cells, are among these.
These new and improved generations of CAR-T cell therapy are promising. However, they are still in the early stages of clinical trials and will need a few years until they can reach the market.
Making cancer technology more precise
CRISPR/Cas9 has changed the field of gene editing by making it much simpler and faster to modify DNA sequences with high precision. One of the first medical applications of this technology could be in cancer.
In China, scientists are using CRISPR gene editing to remove a gene from immune T cells that encodes a protein called PD-1 that tumor cells can use to evade an immune attack. Another trial with a similar approach is currently running in the US.
In addition, CRISPR technology could also be used to improve cancer therapies such as CAR-T.
“The precision and efficiency of gene editing with CRISPR/Cas9 enables the rapid creation of CAR-T cell therapies that may have distinct advantages over the current generation of CAR-T products,” Sam Kulkarni, CEO of the Swiss company CRISPR Therapeutics, told me. A pioneer in CRISPR/Cas9 therapy, the company is behind the first human trial using the technology in Europe, which is intended to treat a genetic blood disorder.
In particular, Kulkarni believes CRISPR/Cas9 has potential in the creation of off-the-shelf CAR-T cell therapy that is sourced from donors. “We make two edits to the donor T-cells. The first edit changes the genetics of healthy donor T-cells so they are programmed to attack only cancer cells versus indiscriminately attacking the patient’s cells. The second edit cloaks the T-cells so they don’t appear foreign to the body and can provide a more durable response,” explained Kulkarni.
The technology is still in its early stages, and there are concerns that the overall effects of using CRISPR in humans are not yet fully understood. But if these concerns are successfully addressed, the potential for cancer is big.
“An allogeneic CAR-T approach using CRISPR/Cas9 gene editing has the potential to transform the way we treat cancer,” said Kulkarni. “This is a scalable approach that can get potentially life-saving treatments to patients with greater speed and efficiency than conventional autologous CAR-T treatments.”
Teaming up with microbes
In the last decade, researchers have uncovered that the human microbiome — the collection of microorganisms that live in our bodies — plays an important role in many aspects of our health. Including cancer.
“Elements of the microbiome play a role in suppressing an overactive immune system in inflammatory diseases, and in boosting a suppressed immune system in cancers,” said Christophe Bonny, CSO of Enterome, a French company developing medicines based on microbiome science.
“Our novel therapeutics are based on our knowledge of the interaction between the immune system and the gut microbiome,” he explained. “We know that tumor cells are often invisible to the immune system. We also know that within the microbiome there are peptides that mimic antigens on the surface of tumors. These can be used to make the tumor visible to the immune system again.”
Enterome has created a cancer vaccine based on these tumor-mimicking molecules, which generates strong immune responses against the tumor. The goal is to reactivate the immune system and make tumors ‘visible’ to other forms of cancer therapy. In particular, Enterome aims to combine this technology with checkpoint inhibitors, a type of drug that blocks one of the mechanisms by which tumor cells hide from the immune system.
Although the cancer vaccine has shown promise in preclinical studies, is still has not been tested in human trials. “It will be several years before it becomes available, assuming it is successful in clinical studies,” said Bonny.
Towards a brighter future
Advances in multiple fronts are increasingly making cancer a more manageable disease. “There are still many unmet needs in cancer, any new and effective treatment will be a positive addition to the oncologist’s arsenal,” said Bonny.
With more options available, patients will be able to get therapies that are specific to their needs. “Everything today is becoming more and more personalized,” said Marett. “With cancer treatments, the genomic information on the tumor is increasingly becoming available as the cost of sequencing continues to drop. We can expect a future where we will have much more precise treatments based upon the genomic signature of the patient.”
He believes that this will deeply change the way companies in oncology operate; “A pharmacy will no longer be selling tablets off the shelf. We’re going to be delivering a specific treatment only for the patient.” With treatments being offered as services rather than products, Marett expects that the relationship between drug makers and patients will change, becoming closer.
Most of these new technologies against cancer still have to prove their worth in clinical trials. Even in the best-case scenario, it will be several years until they are available. However, we are on track to reach a future where cancer treatment is personalized and the chances of beating the disease are higher than ever.
Images via Shutterstock. This article was originally published on October 2018 and has since been updated.
Oncology R&D trends and breakthrough innovations







