Newsletter Signup - Under Article / In Page
"*" indicates required fields
We talked to the CEO of Bicycle Therapeutics, Kevin Lee, about how his company’s technology could cure not just cancer but also a raft of other diseases.
Bicycle Therapeutics made a splashy debut in 2014 with a €25M fundraising round, only to outdo it with a €1B deal with AstraZeneca last fall. What’s the fuss? The company’s bicycle-drug conjugates (BDCs) use bicyclic peptides to guide toxins in an approach similar to antibody-drug conjugates (ADCs); and they stand to outperform their antibody-guided counterparts.
Kevin Lee, CEO of Bicycle Therapeutics, sat down with us to explain more about the technology. A pharmacologist by training, he joined the company in September 2015 after a stint at Pfizer as CSO of the Orphan and Genetic Diseases division.
“I’m really interested in novel disruptive technologies and Bicycle’s idea is very different and very compelling,” he says. “I joined Bicycle because I fell in love with the technology and felt this was the best way I could make a difference.”
While it seems his focus has shifted from rare disease to cancer, Lee stands by his long-standing interest. “Bicycle is focused on cancer for now,” he says, “but we’re going after a much bigger idea.”
So what’s the bigger idea?
So we made the strategic decision to focus on oncology because we’re a small company, and you can’t try to target a bunch of different indications. We’re looking at partnerships in a variety of different areas, but one that stands out to us is respiratory disease.
Bicycles have so much potential here, because they’re small enough that they can be absorbed through inhalation. We are innovating in drug delivery, I suppose, but we’re developing therapeutics — you can always ask, where does one start and another one end?
The point is that bicyclic peptides are very specific, fast-acting and rapidly cleared. They’re also chemically synthesized so we can generate a lot very quickly.
But peptides, from a chemical standpoint, aren’t known for their specificity or ease of synthesis. How do yours overcome these challenges?
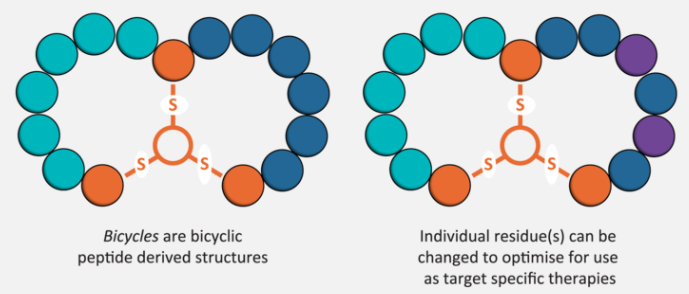
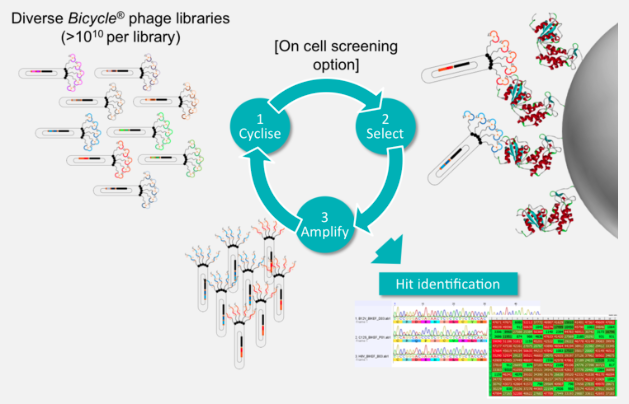
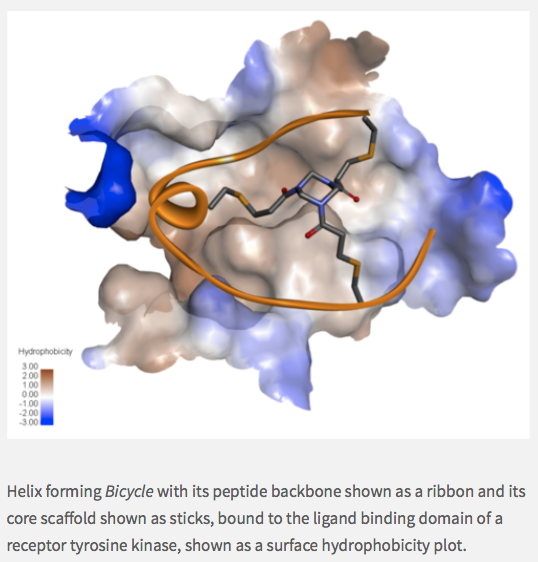
What makes Bicycle unique is that it’s build on the insights of Greg Winter, who came up with the idea of phage display for antibodies. It’s remarkable that at least two thirds of all marketed antibodies can be traced back to his technology, and now we’re using that technology to make bicycle peptides.
So, to put this in perspective, the ability to find novel drugs depends on finding a hit in your chemical library, and this is a function of library size and diversity. If you’re a pharma company, you’ve got a small molecule library; you’re very pleased if you’ve got a million compounds, and you think you’re very good if you’ve got five million…but our bicycle library is several quadrillion molecules — that’s 1015.
To try and put this difference in size into context, imagine each compound is a second of time: five million compounds would be 60 days, and a quadrillion compounds would be 32 million years with phage display…but we can screen the whole library in four to six weeks.
So, we have no problems finding the bicycles and optimizing them for nanomolar potency and high selectivity without ever having to synthesize a peptide. The phage does the work for us, and evolution drives drug discovery. Once we have the peptide, it’s just solid-phase peptide synthesis: in six months, we’ve gone from ten-milligram scale to multi-kilogram scale.
Is that genetic component how it relates to your background in genetic and rare diseases?
I have a long-standing interest in those kinds of disease, and our platform has broad application to many different areas. My background in genetics got me interested in oncology and genetic disease; I see our technology as being great for oncology, rare disease and research into many other diseases.
 Right now, we have to narrow our focus, since we’re a small company, and we’ve chosen oncology. We’ll continue to pursue partnerships outside of oncology, and almost certainly those partnerships will include areas of public health and rare diseases. What we have to do first is develop the technology, get it to the clinic, and create value so we can apply it to other areas.
Right now, we have to narrow our focus, since we’re a small company, and we’ve chosen oncology. We’ll continue to pursue partnerships outside of oncology, and almost certainly those partnerships will include areas of public health and rare diseases. What we have to do first is develop the technology, get it to the clinic, and create value so we can apply it to other areas.
So how exactly do the bicycles work?
We use semi-randomized DNA sequences upstream of the phage gene three, which is a classic gene you attach to peptides you want to synthesize on the surface of the phage — classic biotechnology, really. Our peptides have tunable pharmacokinetics, meaning we can adjust the half-life by modifying peptides with, say, unnatural amino acids.
If we decorate the peptides with carboxylic acids, they can bind to serum albumin, which acts as a reservoir so further extending half life. As the peptides are excreted through the kidneys, the rate of clearance can be a few minutes to a few months. So we can really tune the pharmacokinetics as we’d like.
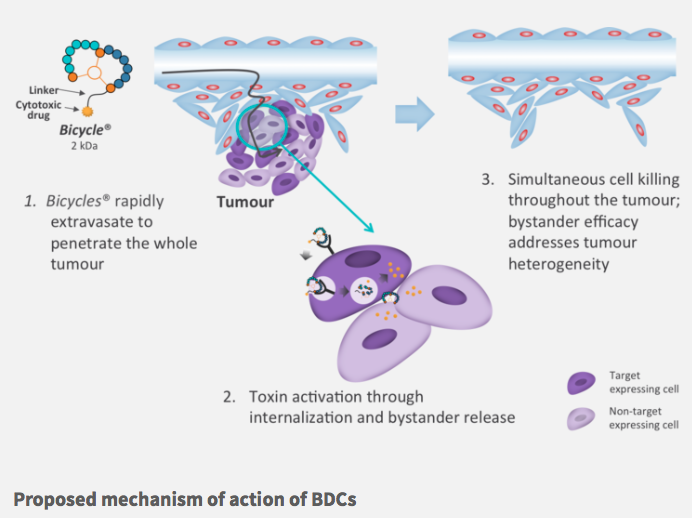
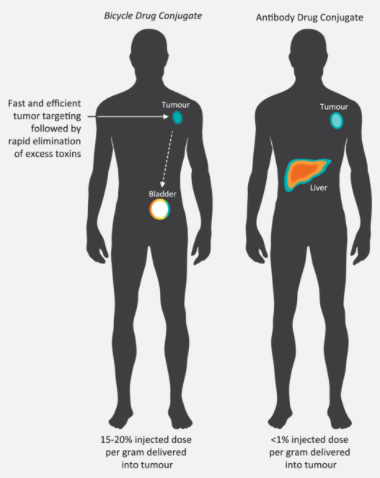
For oncology, we’re developing molecules with a very short half-life of about 20 minutes. The problem with the antibodies in ADC’s is that they have very long half-lives, and they stay in the body for such a long period that you get toxicity and serious side effects.
Also, ADC’s are large and don’t penetrate tissues well, so it’s a bit like peeling an onion as the onion is growing. By contrast, bicycles are small so they can get to the centre of the tumor. I think of it like the Death Star in Star Wars — we’re going right for the middle of the thing to blow it up. We’ve seen that we can eliminate tumors not readily accessible to antibodies — massive tumors — after four to six doses over several weeks, and they don’t come back.
We don’t see ourselves as competing with antibodies though; we’ve identified the niche for bicycles, and while antibodies might be effective treatments for liquid tumors, we’re after the solid ones.
But to run with the comparison to ADC’s, do you run into any problems with ‘de-drugging’ before the bicycle gets to the target?
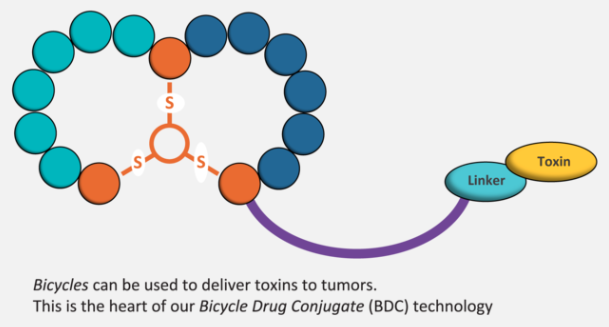
In ADC’s, the linkers are uncleavable; they’re only broken down following internalization and lysozomal processing. But these don’t work with our technology, because our drugs aren’t in the body for long enough. Our BDC’s go into the tumor, bind to the target, stick around for long enough for the toxin to be clipped off and released into the extracellular space. The toxin can then diffuse into its closest cell, independent of the target that’s expressed.
On the other hand, ADCs are limited to targets with rapid internalization, which is a fairly small number. Moreover, tumours are usually heterogeneous, so you can only take out a portion of the tumor. The diffusion of our toxins and the bystander effect means that we can take out the whole thing, which may be why we don’t see relapses.
Thanks so much to Kevin for taking the time to explain all of this! Despite all the fuss about ADCs, it seems like BDCs are just as worth watching. We look forward to following Bicycle’s progress in cancer and beyond!
Images from Bicycle Therapeutics and Titikul_B / shutterstock.com
Oncology R&D trends and breakthrough innovations