Newsletter Signup - Under Article / In Page
"*" indicates required fields
Innate Pharma’s checkpoint inhibitor candidate lirilumab missed its primary endpoint in a Phase II trial, which gave a hard blow to the company’s stock.
When the stock market opened this morning, Innate Pharma‘s stock went down by almost 25%. The company had just announced the results of a Phase II trial that failed to prove its lead candidate lirilumab effective in patients with acute myeloid leukemia (AML).
Innate Pharma, a French biotech specializing in immuno-oncology, licensed lirilumab to Bristol-Myers Squibb back in 2011 in a deal that could reach up to €400M ($430M). The team is currently conducting 6 other clinical trials evaluating the antibody in combination with drugs from BMS to treat several types of cancer.
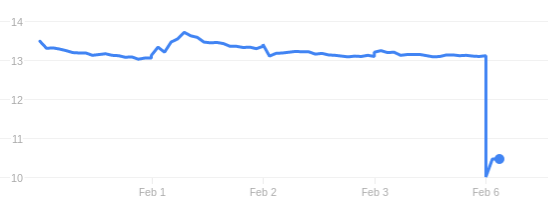
Lirilumab is a checkpoint inhibitor that targets killer-cell immunoglobulin-like receptors (KIRs) in natural killer cells to activate their antitumoral activity. The failed study is the first Phase II trial to be completed with lirilumab, which does not look like a good predictor for the outcome of the other 6 trials.
Although investors have reacted quickly to this morning’s announcement, there’s still hope for the French biotech. Pierre Dodion, CEO of Innate Pharma, commented that the company has seen “early efficacy signals of lirilumab in combination with nivolumab“. These preliminary results come from a Phase I/II combination study aimed at patients with squamous cell cancer of the head and neck (SCCHN), which recently triggered a €14M ($15M) milestone payment from BMS.
Further, Innate Pharma is also running clinical trials with other two checkpoint inhibitors. One of them, monalizumab, is being co-developed in partnership with AstraZeneca. If everything goes well, the deal could reach up to a massive €1.2B ($1.28B).
Images from Argus /Shutterstock, Google Finance
Oncology R&D trends and breakthrough innovations







