Newsletter Signup - Under Article / In Page
"*" indicates required fields
GW Pharmaceuticals, a British biotech developing cannabinoid drugs, is on a high after a successful $300M public offering on the Nasdaq Stock Market.
The company announced the public offering swiftly after its lead cannabinoid drug, Epidiolex, made history by being the first cannabinoid drug to obtain approval from the FDA earlier this year. GW plans to launch the drug before the end of the year in the US as a treatment of the rare forms of epilepsy Lennox-Gastaut syndrome and Dravet syndrome. In Europe, Epidiolex is currently awaiting an approval decision from the EMA, due in 2019.
Epidiolex is a purified solution of cannabidiol, an extract of the cannabis plant. Cannabis is under strict legal restrictions in the US and other countries, so drug derivatives like Epidiolex must jump through more legal hoops to get market approval. However, Epidiolex cleared the final regulatory hurdle in the US last month as the US Drug Enforcement Administration reduced it from the highly restricted Schedule I category of prohibited drugs to Schedule V, the least restricted category.
Unlike cannabis, Epidiolex carries no addictive side effects. This is because its key ingredient, cannabidiol, is not psychoactive. The psychoactive ingredient in cannabis is a chemical called tetrahydrocannabinol (THC). Some FDA-approved therapies such as Syndros from Insys Therapeutics contain synthetic versions of THC, but not extracts from the cannabis plant.
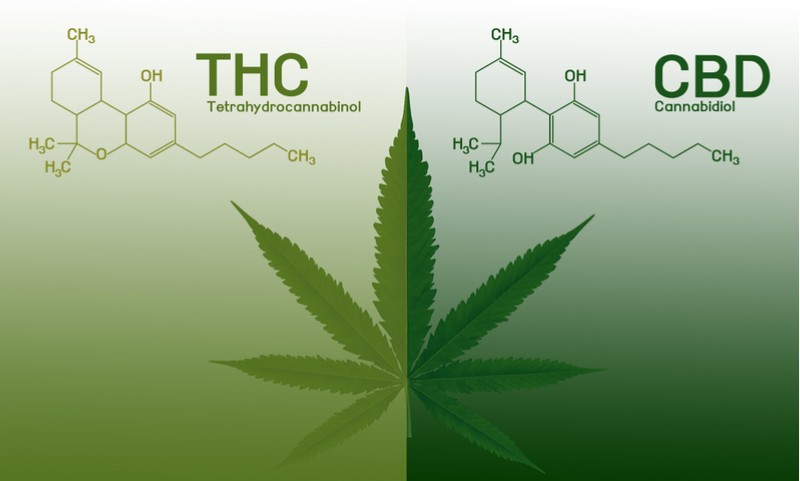
GW also made history in 2016 when its mixed THC and cannabidiol treatment Sativex was the first to enter the market in several countries for the treatment of muscle stiffness in multiple sclerosis. Sativex is now in a Phase III trial in the US to obtain FDA approval for the same indication.
With the legalization of medical cannabis gaining traction in Europe, there is a big market opening up for companies like GW to exploit. A cannabinoid drug made by a US competitor, Zynerba, failed a Phase II clinical trial for the treatment of epilepsy last year, but Zynerba plans to test the drug for treating symptoms of other conditions such as Fragile X Syndrome.
Images from Shutterstock






