News and Trends 3 Aug 2022
Full label approval given by FDA for eye disease drug, CIMERLI
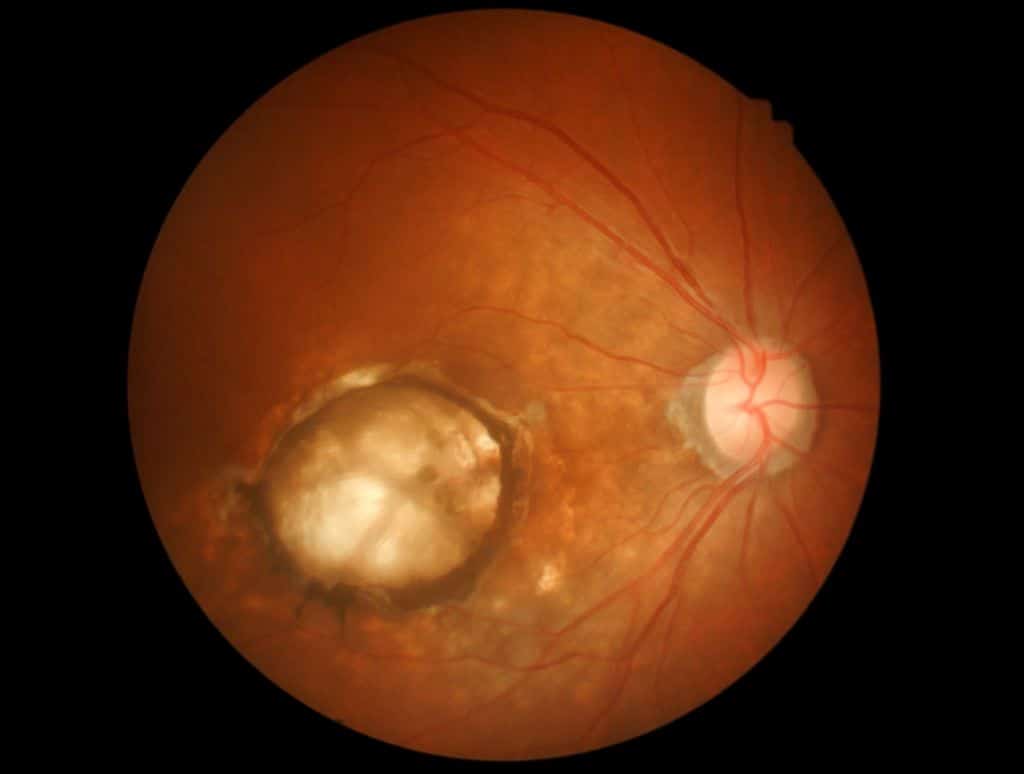
Age-related neovascular (wet) macular degeneration (nAMD) and other serious retinal diseases can now be treated after the U.S. Food and Drug Administration (FDA) approved a biosimilar product interchangeable with an injection. The approval of CIMERLI (ranibizumab-eqrn) interchangeable with Lucentis, a ranibizumab injection was jointly announced by Polpharma Biologics BV, Formycon AG and Bioeq AG today […]