Newsletter Signup - Under Article / In Page
"*" indicates required fields
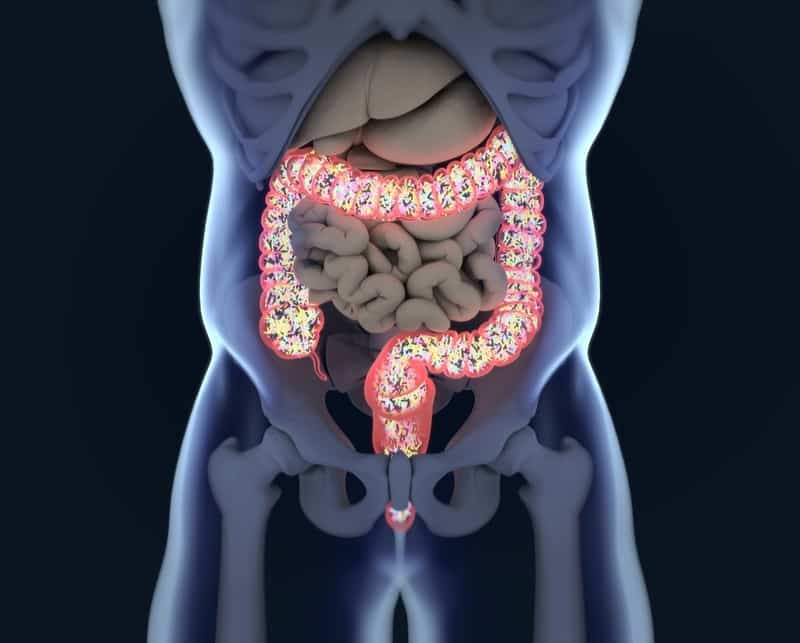
Patients with moderate to severe ulcerative colitis (UC) can now be enrolled onto two phase 3 induction studies for a biotech company’s lead candidate drug.
France-based Abivax SA is a phase 3 clinical stage biotech company that focuses on developing therapies that modulate the immune system to treat chronic inflammatory diseases, viral infections, and cancer.
It received approval from the central U.S. Institutional Review Board (IRB) for the protocols for the studies.
This allows the initiation of enrollment with obefazimod (ABX464) in UC in the U.S. A first-patient-in is anticipated for the end of the third quarter of this year.
Induction studies
Hartmut Ehrlich, CEO of Abivax, said: “We are very pleased with the U.S. IRB approval of the phase 3 induction protocols to confirm efficacy and safety of obefazimod in adults with moderate to severe ulcerative colitis. Enrolment of patients can be initiated for the two induction studies and subsequently for the maintenance trial. Patients and caregivers urgently need alternative therapeutic options for the treatment of ulcerative colitis.
“The obefazimod phase 2b study provides compelling long-term efficacy data demonstrating potential for obefazimod to substantially and durably improve the lives of UC patients in the U.S. and worldwide. Abivax is confident that the phase 3 studies will confirm the positive and promising results of the phase 2a and 2b induction and maintenance trials.”
Supportive information
Following a meeting in late 2021, at the end of the second phase with US regulatory agency, FDA, and scientific advice from European regulatory the European Medicines Agency (EMA), Abivax has submitted final phase 3 protocols and the required supportive information to the U.S. IND in June 2022.
In Europe, the clinical trial applications for the phase 3 protocols will be submitted under the new Clinical Trial Regulation in August 2022 and approval is expected by December 2022.
Didier Blondel, CFO of Abivax, added: “We are pursuing several options for extending our cash runway beyond end of September 2022, taking into account the current fundraising environment for biotech companies. The whole Abivax team is looking forward to the inclusion of the first patient in the phase 3 studies.”
Phase 3 program
The global phase 3 clinical program with obefazimod in ulcerative colitis in 1,200 moderate to severe UC patients across 36 countries will take part in the pivotal phase 3 program, which consists of two induction studies and a subsequent maintenance study.
These three pivotal studies are all randomized, double-blind and placebo controlled, using independent and central review of the video-taped endoscopies.
Abivax plans to post the trial designs as well as participating study sites on clinicaltrials.gov by end of September. In consultation with international regulators, including FDA and EMA, obefazimod 25mg and 50mg will be investigated in phase 3 for the treatment of UC in advanced therapies (AT) naïve and in AT-failure patients to support the future submission of marketing authorizations.
In 2019, an extension of Abivax’s phase 2a trial showed its treatment for the inflammatory bowel disease ulcerative colitis had good efficacy after a year of treatment, with 75% of the screened patients remaining symptom-free.