Newsletter Signup - Under Article / In Page
"*" indicates required fields
Recombinant antibodies have yet to be applied to the treatment of snakebites, a neglected tropical ‘disease.’ Researchers from Denmark investigated the technology and cost of this novel approach in biotech.
Bites from venomous snakes are a major public health problem in tropical areas, where 2 to 3 million people are affected every year and an estimated 100,000 die. Published in PLOS Neglected Tropical Diseases, a study says recombinant antivenoms may be the solution. Andreas Laustsen, co-founder of VenomAb also known as ‘Snake-Bite Jesus’ from Labiotech Refresh, and his coworkers outlined how to make these synthetic antibodies cost-effective via various manufacturing strategies.
Part of the problem with antivenoms is that the development of the science behind them has ground to a halt, and even when logistics run smoothly in their delivery, there may be a lack of good options. The current gold standard is animal-derived serum, which contains a low percentage of antivenom antibodies and carries a high risk of serious allergic reactions. Besides its therapeutic deficiencies, there’s also a current shortage of antivenoms in sub-Saharan Africa.
The team of researchers says biotech is ready to tackle antivenoms and has crunched the numbers to prove it could be cost-effective. The key technological challenge of recombinant antivenoms lies in the diversity of toxins a single snakebite may carry. Additionally, often the species of the “offending snake” can’t be identified, so a universal antivenom for the venomous snake of a given area would be the ideal solution. To target all the common toxins involved, there’s a need for a mixture of antibodies.
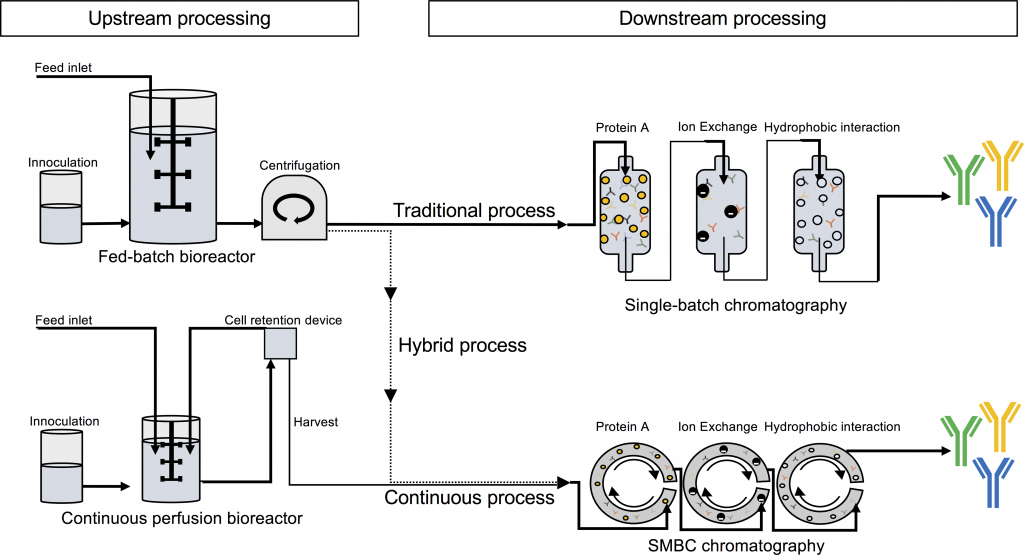
Mixtures of antibodies can be achieved by just mixing batches of good old monoclonal antibodies, but there’s also a new technology emerging for these types of therapeutic products – oligoclonal expression. Biotechs with this second strategy include Netherlands-based Merus and the Danish Symphogen (we recently interviewed one of Symphogen’s founders).
Estimating that there’s a need of 500 kg of active antibodies in sub-Saharan Africa alone, the researchers behind the PLOS study looked at how much it would cost to produce recombinant antivenoms using Symphogen’s platform, Sympress, and different methods of cultivating the CHO cell lines that produce the antibodies. Another factor is the separation during the final stages of bioprocessing. While chromatography is regularly used for antibody products, a method called caprylic acid precipitation is used in current antivenom production and is significantly cheaper.
The final verdict? A dose of recombinant antibodies could cost from €47 to €330, with the cheapest process consisting of a hybrid perfusion process for cell cultivation and caprylic acid precipitation. Compared to the cost of current antivenoms (€52 to €600), there’s still a comfortable margin for all the other logistics required, such as distribution and sales.
Images from Julian W./Shutterstock and Laustsen et. al (2017) Recombinant snakebite antivenoms: A cost-competitive solution to a neglected tropical disease? PlOS Neglected Tropical Diseases (doi: 10.1371/journal.pntd.0005361)
Artificial intelligence and the future of antibody discovery






