Newsletter Signup - Under Article / In Page
"*" indicates required fields
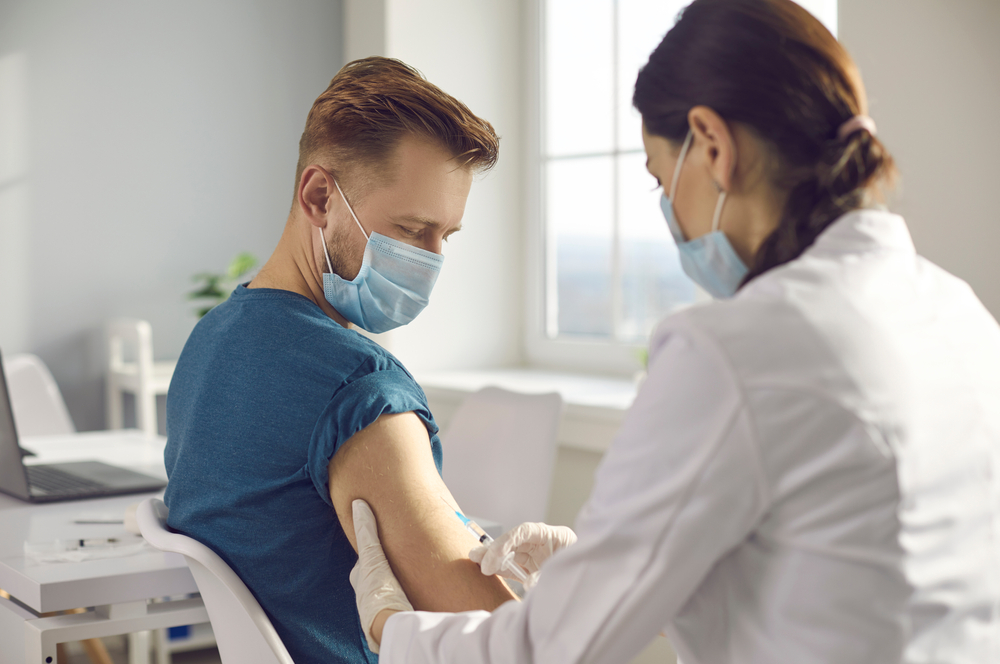
The majority of people in the U.K. (68%) would consider taking part in a clinical trial, it has been revealed today, while half of Brits would also be willing to take part in an early phase clinical trial.
Financial payment is the most popular motivation for taking part in a clinical trial, but discovering new treatments and the importance of clinical research are also top considerations.
The data, released by clinical trials start-up Lindus Health, aims to provide policymakers with an understanding of how to help get the U.K. back on track to fulfill its ambitions to be a life sciences superpower.
Clinical trials in the U.K.
Despite the willingness to participate, more than nine in 10 people living in the U.K. have never taken part in a trial, suggesting there is huge untapped potential for trial recruitment. Key concerns for participants include safety and health, the time and effort required, and a lack of job flexibility. Between 2017 and 2021, there was a 44% drop in participants recruited to commercial clinical trials – which is currently being investigated in an independent government review of the U.K. clinical trials landscape.
Among Black, African and Caribbean respondents, a lack of trust is one of the top barriers to taking part in an early phase clinical trial, yet this group is still more likely to have previously taken part in clinical trials at any stage.
The newly-released data comes as statistics from the Association of British Pharmaceutical Industry show that the number of clinical trials in the UK has plummeted, with the U.K. falling behind globally – especially for phase III trials. The number of trials initiated in the U.K. fell by 41% between 2017 and 2021. In the U.K., fewer phase I trials are carried out than in the U.S., China and Australia. For phase III, the U.K. is lagging behind countries including Spain, France, Germany, Poland and Italy. Cancer trials have seen the biggest fall in numbers in the U.K.
Michael Young, co-founder of Lindus Health, said: “Clinical trials are crucial to the process of discovering cutting-edge treatments that can save lives.”
“The U.K. has the tools to be a life sciences superpower, including citizens willing to participate in clinical research – yet the number of clinical trials is nose diving and nine in 10 people here have never taken part in one.”
“From failing to give participants the financial compensation and flexibility they need, to a cumbersome process that requires far too much time and effort, the old-fashioned clinical trials industry is holding back the development of life-changing treatments.”
About the survey
The survey was carried out online in collaboration with Prolific between March and September 2022. There were 1,049 respondents.
Overall, 91.2% of respondents have never taken part in a clinical trial. However, 68.6% would consider taking part in one, irrespective of the phase. After providing a detailed definition of what an early phase trial entails, 50.2% of responders were willing to consider participation.
Financial payment: a key incentive to take part in clinical trials
Overall, the sample reported financial payment, helping discover new treatments and awareness of the importance of these studies for society as the key incentives to take part in clinical trials.
Other challenges in clinical trial recruitment
There are other challenges in recruiting for clinical trials.
Common barriers precluding participants from partaking in clinical trials included concerns over safety and health outcomes, the amount of time and effort required to take part in one, and not being advised by people around them.
Although somewhat similar for early phase clinical trials, responders reported low monetary reimbursement, instead of not being advised by people around them to be among the top three barriers.
Partnering 2030: The Biotech Perspective 2023