Newsletter Signup - Under Article / In Page
"*" indicates required fields
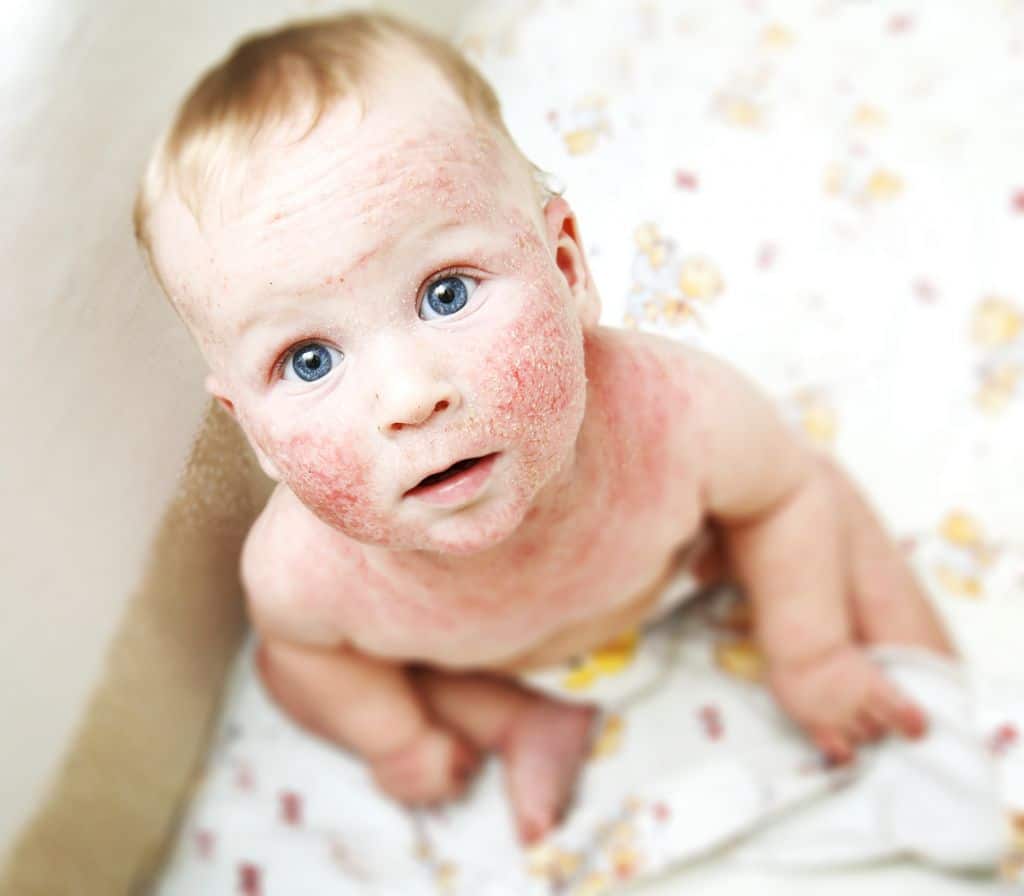
Positive results from a phase 3 trial in children aged 6 months to five years old with uncontrolled moderate-to-severe dermatitis have been published.
Regeneron Pharmaceuticals, Inc. and Sanofi made the announcement today (September 16) that the Lancet published details on the success of Dupixent (dupilumab).
These data were the basis for the U.S. Food and Drug Administration (FDA) approval of Duxipent in June this year (2022) and for a regulatory submission currently under review by the European Medicines Agency (EMA).
Dupilumab is being jointly developed by the two companies under a global collaboration agreement. To date, dupilumab has been studied across more than 60 clinical trials involving more than 10,000 patients with various chronic diseases driven in part by type 2 inflammation.
Atopic dermatitis
Amy Paller, principal investigator of the trial, said: “The Lancet’s publication of these phase 3 results is a testament to the significance of the data showing dupilumab can alleviate the multidimensional burden that moderate-to-severe atopic dermatitis places on infants, toddlers and their families.
“By addressing the key inflammatory pathway driving atopic dermatitis, the trial demonstrated that dupilumab not only addressed debilitating symptoms like persistent itch and skin lesions, but also meaningfully improved sleep and reduced pain – two aspects of daily life that are critical for any child’s development and well-being.”
Data from this trial showed that adding Dupixent to low-potency topical corticosteroids (TCS) significantly improved skin clearance and reduced overall disease severity and itch compared to TCS alone (placebo) at 16 weeks.
Additionally, Dupixent patients experienced significant improvement in measures of sleep quality and skin pain, as well as patient- or caregiver-reported outcomes and health-related quality of life. A substantially lower proportion of Dupixent patients needed rescue medications, compared to those on placebo.
Safety results for Dupixent
Safety results through 16 weeks were similar to the safety profile in patients 6 years and older with atopic dermatitis. Adverse events that were more commonly observed with Dupixent – at 5% or less included conjunctivitis, herpes viral infections, molluscum contagiosum, rhinorrhea and dental caries.
The safety and efficacy of Dupixent in children 6 months to 5 years of age with uncontrolled atopic dermatitis has not been fully evaluated by any regulatory authority outside the U.S.
Atopic dermatitis is a chronic type 2 inflammatory skin disease. Eighty-five to ninety percent of patients first develop symptoms before 5 years of age, which can often continue through adulthood.
The phase 3 randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of Dupixent added to standard-of-care low-potency TCS compared to low-potency TCS alone in 162 children aged 6 months to 5 years with uncontrolled moderate-to-severe atopic dermatitis. Patients treated with Dupixent received either 200 mg or 300 mg, based on weight, every four weeks.
Primary endpoints
The primary endpoints assessed the proportion of patients achieving an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 (almost clear) and at least a 75% improvement in Eczema Area and Severity Index (EASI-75) at week 16.
Children who completed the trial were eligible to enroll in an open-label extension to assess the safety and efficacy of long-term treatment with Dupixent in this age group.