Newsletter Signup - Under Article / In Page
"*" indicates required fields
The European Commission (EC) has expanded the marketing authorization for Dupixent (dupilumab) in the European Union to treat adults with moderate-to-severe prurigo nodularis who are candidates for systemic therapy.
Dupilumab is being jointly developed by Sanofi and Regeneron under a global collaboration agreement. To date, dupilumab has been studied across more than 60 clinical trials involving more than 10,000 patients with various chronic diseases driven in part by type 2 inflammation.
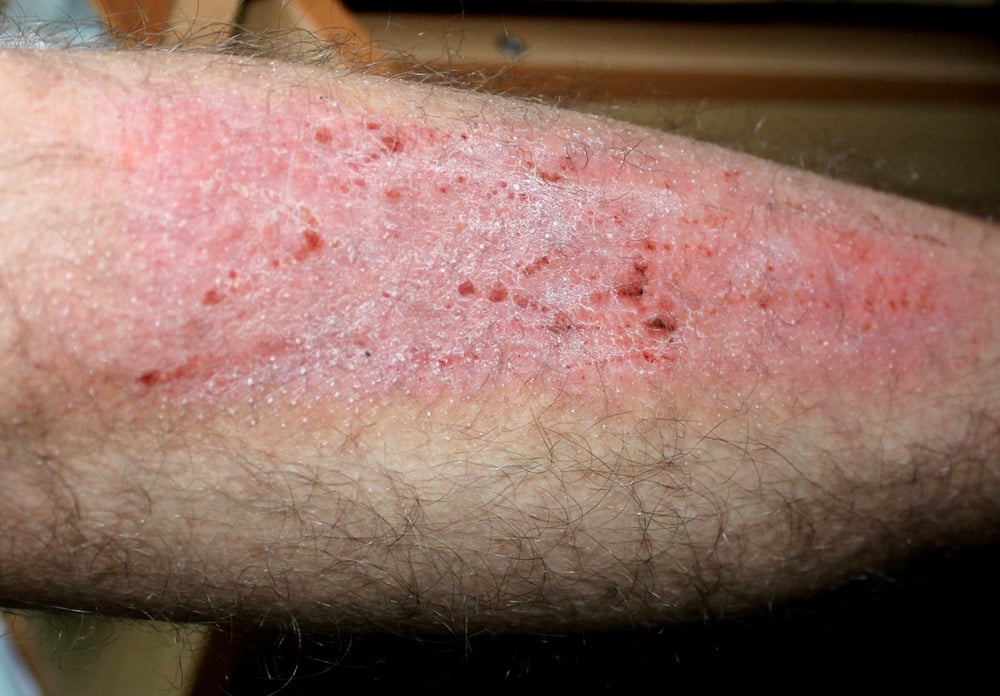
Prurigo nodularis is a chronic, debilitating skin disease with underlying type 2 inflammation and its impact on quality of life is one of the highest among inflammatory skin diseases. With this approval, Dupixent is the first and only targeted medicine specifically indicated to treat prurigo nodularis in Europe and the U.S.
Relieving a burden
Naimish Patel, head of global development, immunology and inflammation at Sanofi, said: “As the first and only targeted medicine approved to treat people living with prurigo nodularis, Dupixent has the potential to transform the standard-of-care for people in Europe living with this debilitating skin disease.
“In the pivotal trials, patients treated with Dupixent experienced significant improvements in key hallmarks of the disease, such as reduction in itch and achieving clearer skin, as well as broader impacts on their daily lives. This approval of Dupixent underscores our continued commitment to bringing Dupixent to patients suffering from chronic skin diseases with underlying type 2 inflammation as quickly as possible.”
George D. Yancopoulos, president and chief scientific officer at Regeneron, added: “For the first time, patients with prurigo nodularis in Europe have a medicine that can help relieve the burden of itchy and painful nodules covering their skin, which can have a devastating impact on their day-to-day lives, both physically and mentally.
“Dupixent is now approved for its second dermatological disease and fourth disease overall. We remain committed to further investigating this innovative medicine for diseases – such as chronic urticarias and chronic obstructive pulmonary disease – in which type 2 inflammation may play a role.”
The EC decision was based on data from two phase 3 trials, where 44% and 37% of Dupixent patients experienced a clinically meaningful reduction in itch at 12 weeks, compared to 16% and 22% for placebo. The improvement further increased at 24 weeks, with approximately three times as many Dupixent patients (60% and 58%) experiencing a clinically meaningful reduction in itch from baseline, compared to placebo (18% and 20%).
In PRIME and PRIME2, more than twice as many Dupixent patients (48% and 45%) also achieved clear or almost clear skin at 24 weeks, compared to placebo (18% and 16%). Dupixent also significantly improved health-related quality of life, while reducing measures of skin pain and symptoms of anxiety/depression from baseline at 24 weeks compared to placebo.
Dupixent was granted an additional one-year marketing protection in the EU based on recommendation by the CHMP that the medicine brings significant clinical benefit compared to existing therapies for patients with prurigo nodularis.
About prurigo nodularis
Prurigo nodularis is a chronic, debilitating skin disease with underlying type 2 inflammation and has one of the highest impacts on a patient’s quality of life among all inflammatory skin diseases dues to the extreme itch it causes. Those with prurigo nodularis experience intense, persistent itch with thick skin lesions (called nodules) that can cover most of the body.
The disease is often painful, with burning, stinging and tingling of the skin and can negatively affect mental health, activities of daily living and social interactions. High-potency topical steroids are commonly prescribed but are associated with safety risks if used long-term. In Europe, about 70,000 adults living with prurigo nodularis are most in need of new treatment options.
About Dupixent
Dupixent is an injection under the skin (subcutaneous injection) at different injection sites. In the EU for adults with prurigo nodularis, Dupixent is administered at 300 mg every two weeks, following a loading dose. It is available as both a pre-filled pen and pre-filled syringe at the 300 mg dose. Dupixent is intended for use under the guidance of a healthcare professional and can be given in a clinic or at home by self-administration after training by a healthcare professional.
Dupixent is a fully human monoclonal antibody that inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways and is not an immunosuppressant. The Dupixent development program has shown significant clinical benefit and a decrease in type 2 inflammation in phase 3 trials, establishing that IL-4 and IL-13 are key and central drivers of the type 2 inflammation that plays a major role in multiple related and often co-morbid diseases. These diseases include approved indications for Dupixent, such as atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis (CRSwNP) and prurigo nodularis, as well as investigational disease eosinophilic esophagitis (EoE) in the EU.
Dupixent has received regulatory approvals in one or more countries around the world for use in certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis (CRSwNP), eosinophilic esophagitis (EoE) or prurigo nodularis in different age populations. Dupixent is currently approved for one or more of these indications in more than 60 countries, including in Europe, the U.S. and Japan. More than 500,000 patients have been treated with Dupixent globally.
Dupixent development program
In addition to the currently approved indications, Sanofi and Regeneron are studying dupilumab in a broad range of diseases driven by type 2 inflammation or other allergic processes in phase 3 trials. These include pediatric EoE, hand and foot atopic dermatitis, chronic inducible urticaria-cold, chronic spontaneous urticaria, chronic pruritus of unknown origin, chronic obstructive pulmonary disease with evidence of type 2 inflammation, chronic rhinosinusitis without nasal polyposis, allergic fungal rhinosinusitis, allergic bronchopulmonary aspergillosis and bullous pemphigoid.
These potential uses of dupilumab are currently under clinical investigation, and the safety and efficacy in these conditions have not been fully evaluated by any regulatory authority.
Are you interested in antibody therapy R&D?