Newsletter Signup - Under Article / In Page
"*" indicates required fields
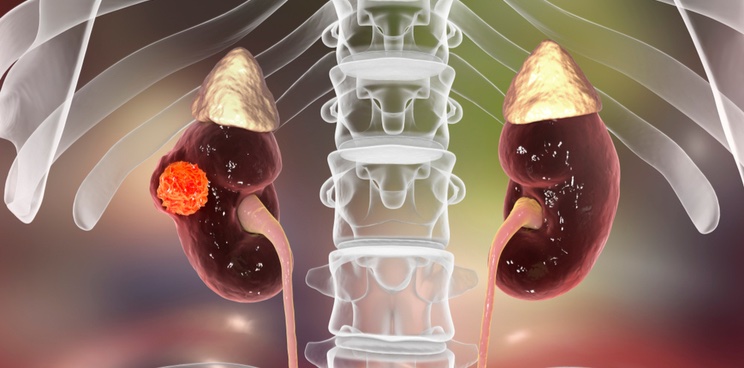
The Swedish biotech Elypta has raised a seed round worth €6.1M to fund a clinical trial of a urine test for signs of recurrent kidney cancer, which could improve patient follow-up after surgery.
The seed round was led by the Swedish VC firm Industrifonden. Other investors in the round included the VC impact fund of the Swedish Norrsken Foundation, Spanish seed fund Nina Capital and Swedish investor Chalmers Ventures.
Along with a €2M grant from Horizon 2020 awarded last year, the proceeds will fund an ongoing clinical trial of Elypta’s urine test, which is designed to detect early recurrences of cancer in patients with clear cell renal cell carcinoma. The trial is expected to finish in 2022, and Elypta aims to launch the test in the US and EU in the same year.
After receiving surgery to remove tumors, kidney cancer patients can undergo routine CT scans to check for recurrences. However, these scans are used infrequently as they can be costly, long, and expose patients to high levels of radiation. Elypta aims to develop the first FDA-approved urine test for this condition, which could allow a cheap way to screen patients more regularly.
“Prognosis is very poor if a tumor recurs after surgery. By providing a safe urine test which can be used more frequently than CT scans, we hope to enable earlier detection and ultimately open a window for curative treatments,” stated Elypta’s CEO, Karl Bergman.
The test looks for signs of kidney cancer by detecting the levels of molecules called glycosaminoglycans in a urine sample. In many types of cancer, these molecules are produced in different quantities to those in healthy people, forming a ‘signature’ that can be picked up by the test. Elypta is also testing its technology to detect other types of urinary tract cancers such as bladder cancer and prostate cancer.
Liquid biopsy in cancer is a growing field. This technique involves tests on body fluid such as urine and blood, and is less invasive than taking tumor biopsies. For example, the UK company Angle is expecting the approval this year of a breast cancer blood test that captures circulating tumor cells in the blood. Another UK company, Cambridge Cancer Genomics, aims to use AI to personalize cancer treatments depending on the DNA shed by a tumor into the blood.
“There are lots of exciting developments in liquid biopsies with a variety of methods where next-generation sequencing is obviously a significant trend,” Bergman told me. “I think there will be more algorithm-based tests and efforts to combine biomarkers. We hope to play a part in this and help add metabolism-based biomarkers to those generated from proteomics and genetics.”
Images from Shutterstock
Oncology R&D trends and breakthrough innovations