Newsletter Signup - Under Article / In Page
"*" indicates required fields
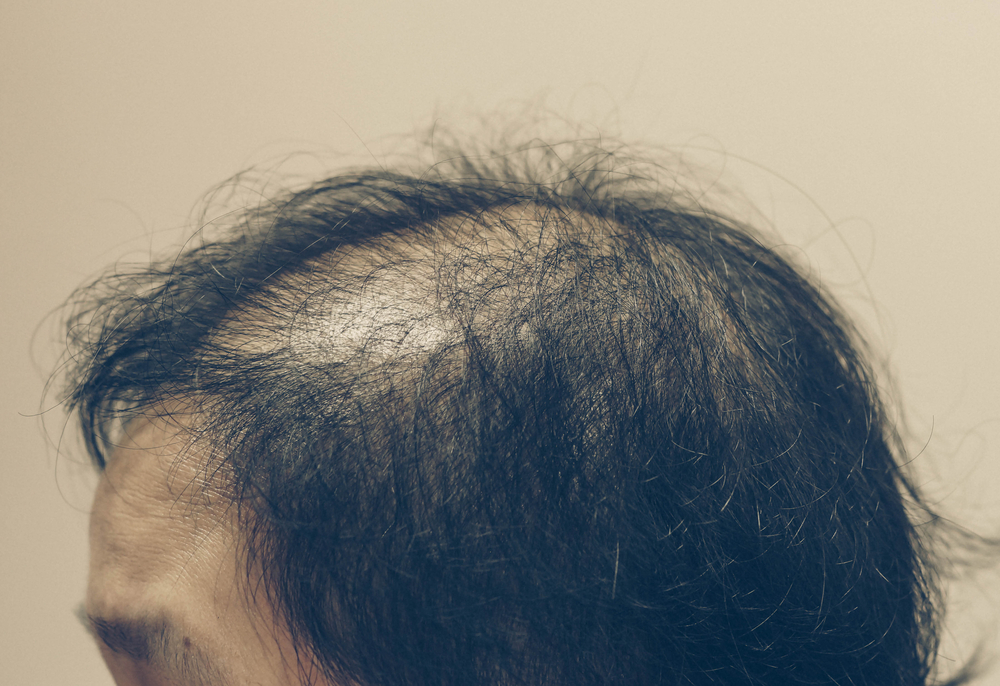
Kintor Pharmaceutical Limited has announced results of a phase II clinical trial of pyrilutamide (KX-826), a potential first-in-class topical drug, for the treatment of adult female androgeneticalopecia (AGA).
The company said in the study KX-826 demonstrated clinically meaningful and statistically significant improvement in hair growth as measured by target area non-vellus hair count (TAHC). In addition, its safety profile was favorable.
This trial is a multi-center, randomized, double-blind, placebo-controlled study designed to evaluate the efficacy and safety of KX-826 for the treatment of AGA in female adults.
Jianzhong Zhang, chairman of the Department of Dermatology, Peking University People’s Hospital, is the leading principal investigator. The primary endpoint for the trial is the change from baseline versus placebo in TAHC at the end of week 24.
A total of 160 female AGA patients who have met Savin Scale (D3-D6) were enrolled in the phase II clinical trial, with 119 patients randomly assigned to four treatment groups.
Pivotal study
Zhang said: “AGA is the most common type of hair loss greatly affecting male and female adults. The market potential for unmet medical needs is huge. In particular, treatment options for female AGA adults are more limited than those for male AGA adults. We are pleased to announce that the first-in-class topical drug, KX-826, has shown a good efficacy and safety profile in the female AGA phase II trial. This result paves the way for the pivotal study which would kick off very soon. Meanwhile, the enrollment of pivotal study of KX-826 for treating male AGA adults is ongoing in China. We look forward to KX-826’s commercialization to provide benefits to both male and female AGA adults worldwide.”
Youzhi Tong, founder, chairman and CEO of Kintor Pharma, said compared with the clinical trial design for male AGA adults study, it is more challenging for the phase II clinical trial of treating female AGA adults, due to changes in their hairline shape, hair loss rating and hair density and lack of detectable biomarkers.
He added that Minoxidil and Finasteride are available for male AGA adults, however, for female AGA adults, the topical treatment is limited to Minoxidil only.
“Therefore, the market size of treating female AGA is large in both China and overseas. We expect to kick off the pivotal study in China soon. In the meantime, we will continue to actively look for partners to expand into the international market. We hope that KX-826 would be an effective and safe first-in-class drug for male and female AGA adults around the globe as soon as possible,” Tong said.
About KX-826
KX-826 is an androgen receptor (AR) antagonist and a potential first-in-class topical drug for the treatment of AGA and acne vulgaris. For the AGA indication, in September 2021, Kintor Pharma announced the primary endpoint of the phase II clinical trial of KX-826 on male adults was met, with results demonstrating a positive efficacy and safety profile.
Kintor Pharma is continuing to conduct a phase III clinical trial of KX-826 in China, and has completed the enrollment of patients in its phase II clinical trial of KX-826 in the U.S. for male AGA adults. For the acne vulgaris indication, Kintor Pharma has completed the enrollment of patients in its phase II clinical trial of KX-826 in China.