Newsletter Signup - Under Article / In Page
"*" indicates required fields
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion, recommending the approval of Dupixent (dupilumab) in the European Union (EU) to treat adults with moderate-to-severe prurigo nodularis who are candidates for systemic therapy.
Dupilumab is being jointly developed by Sanofi and Regeneron.
The European Commission is expected to announce a final decision on the Dupixent application in the coming months. In September 2022, Dupixent was approved by the U.S. Food and Drug Administration for the treatment of adult patients with prurigo nodularis.
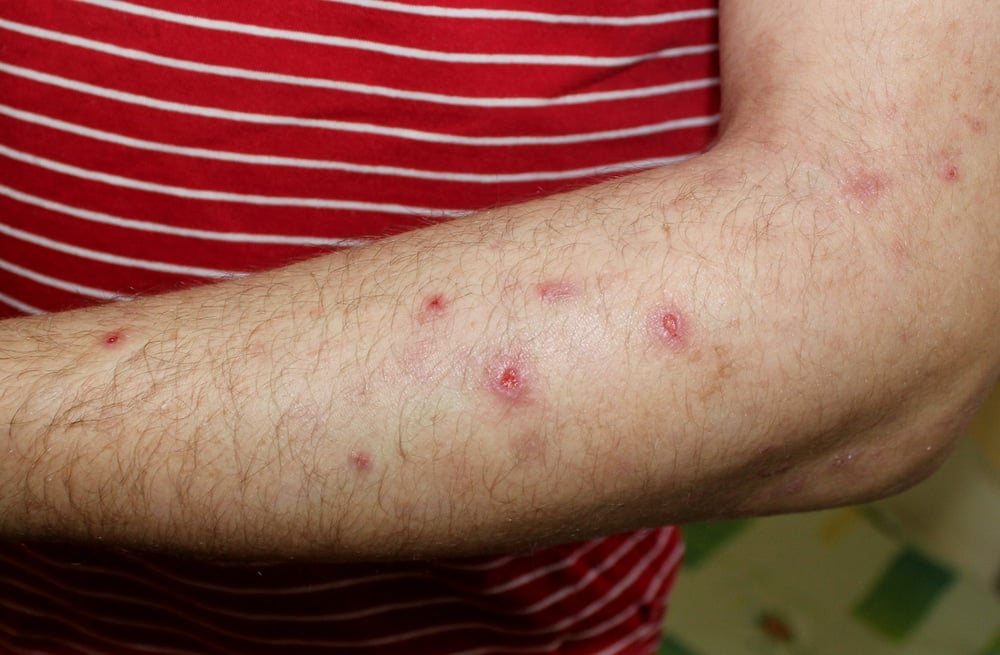
Prurigo nodularis
Prurigo nodularis is a chronic, debilitating skin disease with underlying type 2 inflammation and has one of the highest impacts on a patient’s quality of life among inflammatory skin diseases due to the extreme itch it causes. Those with prurigo nodularis experience intense, persistent itch with thick skin lesions (called nodules) that can cover most of the body.
The disease is often painful – with burning, stinging and tingling of the skin – and can negatively affect mental health, activities of daily living and social interactions. High-potency topical steroids are commonly prescribed but are associated with safety risks if used long-term.
The positive CHMP opinion is supported by data from two phase 3 trials, showing Dupixent significantly reduced itch (the primary endpoint) and skin lesions compared to placebo. Dupixent also significantly improved health-related quality of life while reducing measures of skin pain and symptoms of anxiety/depression. The safety results of the trial were generally consistent with the known safety profile of Dupixent in its approved dermatology indication. Adverse events more commonly observed with Dupixent compared to placebo included conjunctivitis.
The use of Dupixent in adults with moderate-to-severe prurigo nodularis is investigational in the EU and is not yet approved.
About Dupixent
Dupixent is a fully human monoclonal antibody that inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways and is not an immunosuppressant. The Dupixent development program has shown clinical benefit and a decrease in type 2 inflammation in phase 3 trials, establishing that IL-4 and IL-13 are key and central drivers of the type 2 inflammation that plays a major role in multiple related and often co-morbid diseases.
These diseases include approved indications for Dupixent, such as atopic dermatitis, asthma and chronic rhinosinusitis with nasal polyposis (CRSwNP), as well as investigational diseases prurigo nodularis and eosinophilic esophagitis (EoE) in the EU.
Dupixent has received regulatory approvals for use in certain patients with atopic dermatitis, prurigo nodularis, asthma, CRSwNP or EoE in different age populations. Dupixent is currently approved across these indications in the U.S. and for one or more of these indications in more than 60 countries, including in the EU and Japan. More than 500,000 patients have been treated with Dupixent globally.
Dupilumab development program
Dupilumab is being jointly developed by Sanofi and Regeneron under a global collaboration agreement. To date, dupilumab has been studied across more than 60 clinical trials involving more than 10,000 patients with various chronic diseases driven in part by type 2 inflammation.
In addition to the currently approved indications, Sanofi and Regeneron are studying dupilumab in a broad range of diseases driven by type 2 inflammation or other allergic processes in phase 3 trials, including pediatric EoE, hand and foot atopic dermatitis, chronic inducible urticaria-cold, chronic spontaneous urticaria, chronic pruritus of unknown origin, chronic obstructive pulmonary disease with evidence of type 2 inflammation, chronic rhinosinusitis without nasal polyposis, allergic fungal rhinosinusitis, allergic bronchopulmonary aspergillosis and bullous pemphigoid.
These potential uses of dupilumab are currently under clinical investigation, and the safety and efficacy in these conditions have not been fully evaluated by any regulatory authority.
Are you interested in antibody therapy R&D?