Newsletter Signup - Under Article / In Page
"*" indicates required fields
A large clinical study is now beginning on an approved drug for treating psoriasis. The drug will be tested on patients who were recently diagnosed with type 1 diabetes. The theory is that the drug could preserve the patient’s remaining insulin production.
Many hospitals in Sweden are participating in the project, which is funded by the Swedish Research Council. The project is headed by Marcus Lind, a professor of diabetology at the University of Gothenburg and chief physician responsible for clinical diabetes research at Sahlgrenska University Hospital/Östra Hospital and NU-hospital Group.
Lind said that the study could mean a big change in how type 1 diabetes is treated.
“Of the mechanisms now being investigated for immunological treatment of type 1 diabetes, I have the greatest confidence in this one, but I am well aware of how difficult success will be.”
Type 1 diabetes: an immunological disease
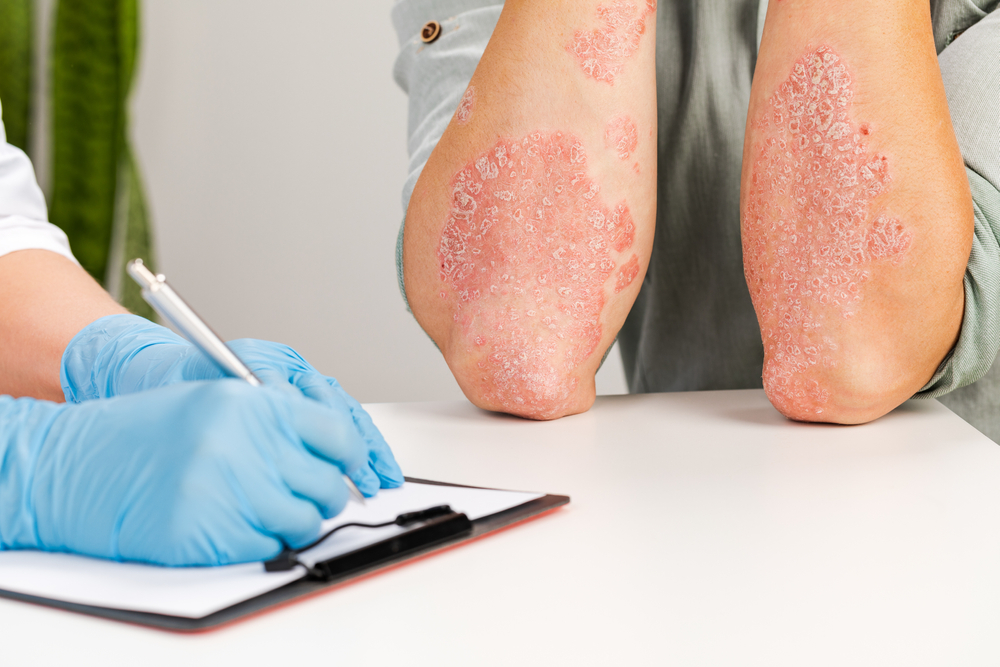
Type 1 diabetes is one of the most common chronic diseases in children, but the disease can also develop in adults. In the disease, the body’s own immune response destroys beta cells in the pancreas so that the body can no longer produce insulin. People with type 1 diabetes need to take insulin injections or use an insulin pump and strictly tract their blood sugar levels for the rest of their lives, which requires a lot of effort.
The beta cells die slowly, so everyone with newly developed type 1 diabetes continues to produce insulin during the first years of the disease.
“They benefit greatly from the remaining insulin their body produces. If they could just maintain this production, treating type 1 diabetes would be much easier. So far, we have not had a good treatment for preventing beta cell death, but we have reason to believe that a medication currently approved for individuals with psoriasis could have a protective effect for individuals with type 1 diabetes,” Lind said.
Tests show stages of the disease
Based on immunological patterns in the blood, currently researchers can determine with a high probability who will develop type 1 diabetes (stage 1 of type 1 diabetes) within a few years. About a year before the onset of the disease, they can then see disturbances in the blood sugar pattern with stress tests, even if criteria for diabetes are not met (stage 2 of the disease). When clinical onset occurs, it is classified as stage 3.
“If we succeed in identifying the immunological mechanism that is central to the destruction of beta cells, we will also be able to screen children and adults in the future and treat them even before the onset of the disease. The disease will then be prevented from breaking out or counteracted so that it does not begin until much later in life,” Lind added.
Psoriasis and type 1 diabetes
The drug to be tested affects the immune response in the body by inhibiting the protein interleukin-17, which seems to be an important signaling molecule in the process that destroys beta cells. For the last few years, the drug has been used as a treatment for psoriasis, where a specific type of white blood cells, known as TRM cells, plays a key role in development of the disease, just as these cells seem to do in type 1 diabetes.
Among other things, these cells act through IL-17, which the current treatment affects.
“In fact, research on type 1 diabetes and IL-17 has been going on for almost 20 years. Animal experiments have shown that stimulation of this signaling pathway accelerates the development of type 1 diabetes. Other studies have shown that this signaling pathway is usually overactivated in people with type 1 diabetes. It will be particularly interesting to evaluate for the first time whether the treatment can protect insulin-producing cells in the pancreas, in light of recent research on TRM cells in newly diagnosed type 1 diabetes, just as in psoriasis,” Lind noted.
Recruitment of individuals with type 1 diabetes for a comprehensive multicenter study has now begun. The study will include adults, between ages 18 and 35, who have been diagnosed with type 1 diabetes in the last three months, where a blood test has shown that they have an ongoing immunological process affecting the beta cells. A total of 127 individuals will be included, with half being randomly assigned to receive IL-17 inhibitors and half receiving a placebo in the control group.
Towards precision medicine
In parallel with the Swedish study now being conducted from the University of Gothenburg, several other studies are underway elsewhere in the world. They are also seeking ways to treat the immunological cause behind type 1 diabetes and not just the symptoms, which until now has been the only treatment option.
As with many other diseases, researchers of type 1 diabetes are now on the verge of precision medicine. Efforts to map different subgroups within type 1 diabetes have just begun to study, for example, a certain gene variation that causes a certain type of islet cell antibodies to first appear.
“It is likely that treatment with IL-17 inhibitors may be more effective for certain subgroups. If the results from our study are encouraging, over time we can investigate certain immunological patterns or cell types in the blood that can be used to identify patient groups that respond best to the treatment,” Lind concluded.
Partnering 2030: The Biotech Perspective 2023