Libra Therapeutics is a San Diego-based biotech focused on developing novel disease-modifying therapeutics, which restore the cellular balance that is disrupted in neurodegenerative diseases.
Established in 2020, Libra is leveraging proprietary technologies and therapeutic assets. The Libra portfolio includes three small molecule programs, including their lead non-clinical program targeting TRPML1 (based on an innovative assay generated by the contract research organization Axxam and hit compounds identified through multiple screening campaigns), which is on track to start Phase 1 clinical trials in 2025, states Martin Gill, Libra’s vice-president and head of biology.
Libra is a completely virtual company, based on a “plug and play concept and mode of operation”, Gill explains. With no in-house laboratory space, the company works closely with multiple international contract research organizations (CROs), including Axxam, which carry out all of the Libra R&D, from compound screening assays, to in vivo pharmacokinetic/pharmacodynamic and ADME work, as well as in vitro and in vivo pre-clinical safety assessments. “When we started Libra it was always the idea that we will bring in different functionalities as required through partnership with CROs,” said Gill, who oversees the Libra R&D workflow from target discovery through the prospective Phase 1 clinical trials.
This virtual biotech set-up brings with it the need for a secure data management platform that can store diverse screening, chemistry and biology datasets from CRO partners, but also gives Libra users easy access, visualization and comparative tools for data retrieval, review, and analysis across disciplines.
Key to effective collaboration with CROs
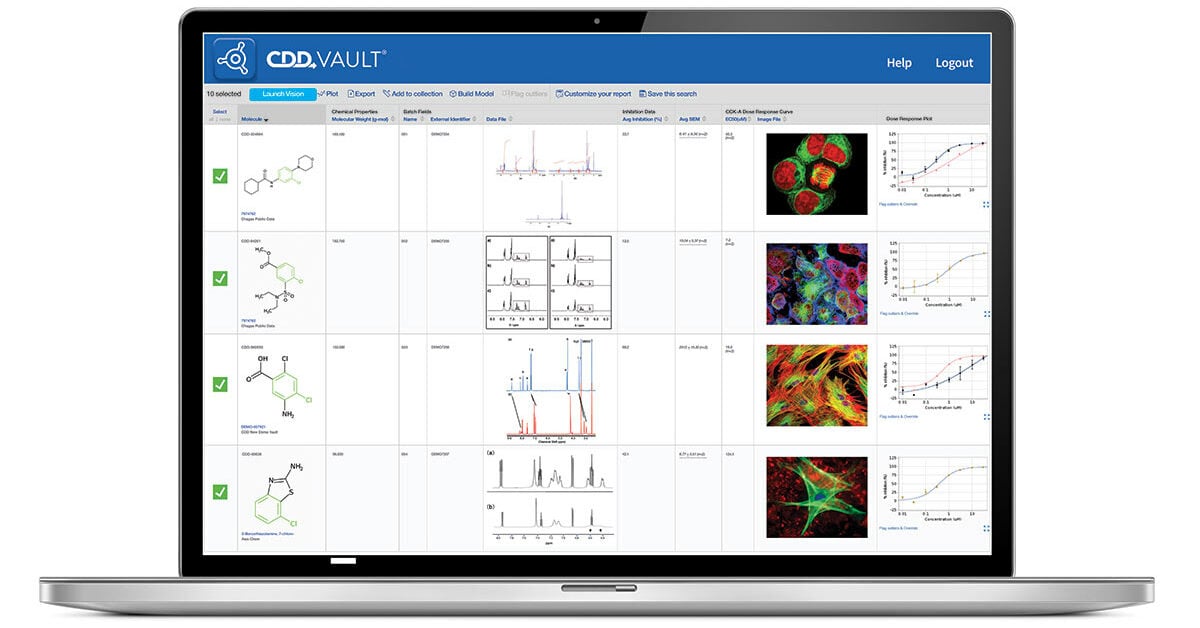
Since its inception, Libra has used CDD Vault as its primary repository for all compound structural data and screening assay results, along with physicochemical and other in vitro biology data and associated metadata generated by its CRO partners.
Selection of CDD Vault as the best fit for Libra was based largely on prior experience. “Libra head of chemistry, Guy Breitenbucher, had previously worked with CDD Vault at different organizations, and was confident that the system would tick all of Libra’s boxes for early hit-to-lead drug discovery programs,” says Gill, who also has extensive experience with a wide range of data management systems, including custom built platforms and commercial offerings. “From my perspective, the Vault provides the high level of functionality and flexibility that might be expected from some of big pharma’s in house platforms, while remaining within the financial reach of smaller biotech companies and academic spinouts.” Key Vault functionality for Libra is the ability to link chemistry to biology. “Using CDD Vault we can maximize the value of the data that we have, to help identify and visually drive SAR, and make good hit-to-lead decisions,” Gill noted. “That decision making is absolutely critical.”
Libra has a close collaboration with Axxam as one of its key CRO research partners. “They’ve got great experience running assays across multiple different target types,” Gill notes.
Dr. Serena Meini, an experienced medicinal chemist at Axxam, has primary responsibility for managing the Libra database, coordinating with CROs to ensure the results and metadata are delivered in the correct formats, uploading all data into the Libra Vault and ultimately ensuring fidelity of the whole process. “Serena is intimately familiar with CDD Vault, and as a chemist, is ideally placed to understand the data, and ensures that all codes and structures are appropriately assigned,” Gill explains.
Working with CROs in Europe, the UK, the US, Canada, India, China and Japan, “We are now also starting to work with clinical and non-clinical DMPK specialists and have engaged a CMC manufacturer, as we move towards API manufacturing for clinical trials with our lead compound. Similarly, toxicology specialism has been brought on board to lead IND-enabling studies,” Gill adds.

Targeting the central nervous system
Libra’s pipeline targets mechanisms that can mitigate protein pathology associated with CNS degenerative disorders. The lead program is focused on identifying small molecule activators of TRPML1 for the treatment of CNS degenerative disorders, as well as degenerative peripheral indications. TRPML1 is a lysosomal cationic channel, which has been shown to induce both lysosomal biogenesis and macroautophagy, processes known to exhibit dysfunction in multiple CNS degenerative indications. “It’s a very hot topic, and a pretty active target and discovery space,” Gill stated.
IND-enabling studies for Libra’s lead TRPML1 program are projected to start during the first half of 2024, with clinical Phase 1 single ascending dose and multiple ascending dose studies on track for 2025, Gill explains. Libra also has two additional discovery-phase programs that are at the target validation stage. “These don’t yet necessitate a data management database such as CDD because there is no established screening system in place for QC-ing those datasets,” Gill notes.
Libra’s discovery and lead optimization workflow for the TRPML1 program is founded on a combination of what Gill describes as reference pharmaceutical assays, including FLIPR (fluorescent imaging plate reader), and high content imaging assays. It’s these routine screening assays – “… many of which typically generate results that can be expressed as a percentage of a control,” Gill suggests – that form the foundation of Libra’s day-to-day research base. “CDD Vault is enabling us to more seamlessly drive our structure activity relationship (SAR) knowledge and understanding through hit-to-lead, and optimize as far as we can through in vitro assay workflows.”
The flexible Vault setup allows users to visualize and compare diverse datatypes in whichever way they need, Gill suggests. “User-friendly access is a significant plus point, and the Vault architecture lets us customize to suit different datatypes, so we don’t have to try to shoehorn our data into a rigid database or engineer complex, expensive workarounds. Using CDD Vault we can, for example, upload our biology results, extract the endpoints that we need, and have all the underlying data – for example, structural chemistry datasets – accessible alongside those biology results and endpoints.”
Having the ability to see the chemistry and biology on the same page means Libra can then more easily and directly relate disciplines. “This helps us to understand which key qualities can be engineered into molecules to drive potency and optimize ADME. That can be really instrumental from an SAR, hit-to lead discovery point of view. By looking at our data from different perspectives, as well as in combination, we can make informed decisions going forward,” Gill points out.
The Vault has other features that have proven a real boon to Libra. “A major plus point is that CDD Vault also creates SAR tables automatically, taking out the need to manually export data to build SAR tables, saving time and potential errors. And we can add features and color coding to aid visualization and intuitive data handling.” Other features help users better understand data anomalies. “We really value the Vault’s ‘notes’ functionality, which lets us flag up and make comments on assay runs that may have thrown up anomalous results, perhaps because control compounds are not behaving as expected. Using notes in CDD Vault we can highlight any screamers in the multiple different runs that have been carried out on a compound, and that lets us understand why the collective assay data is then skewed from the expected.”
Security built in to CDD Vault is critical when working with multiple CROs. Access to the Vault can be restricted by authorization for Libra users as well as for external users, which, Gill notes “protects the data from the potential for accidental internal harm,” as well as from any external security risk. Users can also export data into Excel, so outgoing files can be password protected.
Libra has initiated several confidential discussions with potential clinical development partners for its TRPML1 program, and the flexibility of CDD Vault will prove invaluable, Gill suggests. “The Vault gives us the ability to select, pull up, and organize exactly the data that will be needed for these discussions, tailored to what each potential partner may want to see and understand. The user interface makes it easy to present the data in ways that are informative, and we can also generate customized, as well as standardized reports.”
Enabling clinical hypothesis testing
Industry understandably retains IP close to its chest, but Gill maintains that, based on what’s available in the public domain, the Libra TRPML1 program is the first to have demonstrated in vivo pathway engagement both in the brain and the periphery. “Libra, I believe, has the opportunity with its program to be first-in-class, best-in-class,” he suggests.
Importantly, Libra’s goal is very much biomarker-oriented. “In the neuroscience field particularly, preclinical models are not good predictors of clinical efficacy,” Gill pointed out. “So for us at Libra, we are focused less on being able to cure Alzheimer’s disease in a mouse. Our aim is more about being able to test the biological hypothesis in patients.” To this end, Libra is establishing blood-based biomarkers and linking those to tissue-based biomarkers, so that data from early clinical trials could then inform which indications will likely be successful at “far more expensive” Phase IIb and Phase III trials. “We want to know, with these biomarkers as indicators, whether we can see the movement that we want to see with respect to disease-based endpoints.”
It’s a work in progress, but Libra has identified blood-based biomarkers that are promising from both cellular and organ physiology perspectives. “Our goal is to be biomarker-enabled both from proof-of-mechanism, as well as in terms of potential liabilities.” That is one differentiating feature of Libra’s approach, Gill suggests. “It’s the potential, for each human patient, to define what is the efficacious dose, but also potentially provide signals of risk for potential untoward effects. Being able to do that in blood will open the way to developing a meaningful in-person therapeutic index.”
Initial indications for its lead TRPML1 program will likely be ALS and Parkinson’s disease, although Gill believes there is much wider potential. “There may be an opportunity to target a more niche, peripheral disease with a smaller patient cohort to achieve Phase II line-of-sight faster and demonstrate and confirm that our biomarkers are truly predictive,” Gill noted. “Our goal is to enable clinical hypothesis testing.”
Do you want to learn more about how Libra Therapeutics is restoring cellular balance in the CNS? Or why not discover more about how CDD Vault is making data management easy? Check out the companies’ websites for more information!
Images Courtesy: Collaborative Drug Discovery, Axxam