Newsletter Signup - Under Article / In Page
"*" indicates required fields
ArGEN-X from Ghent (BE) and Breda (NL) has initiated its phase I human trials for Auto-immune diseases such as systemic lupus erythmatosus (SLE) – a rare chronic disease which has had little progress in the last 50 years. Here is the story behind SLE…
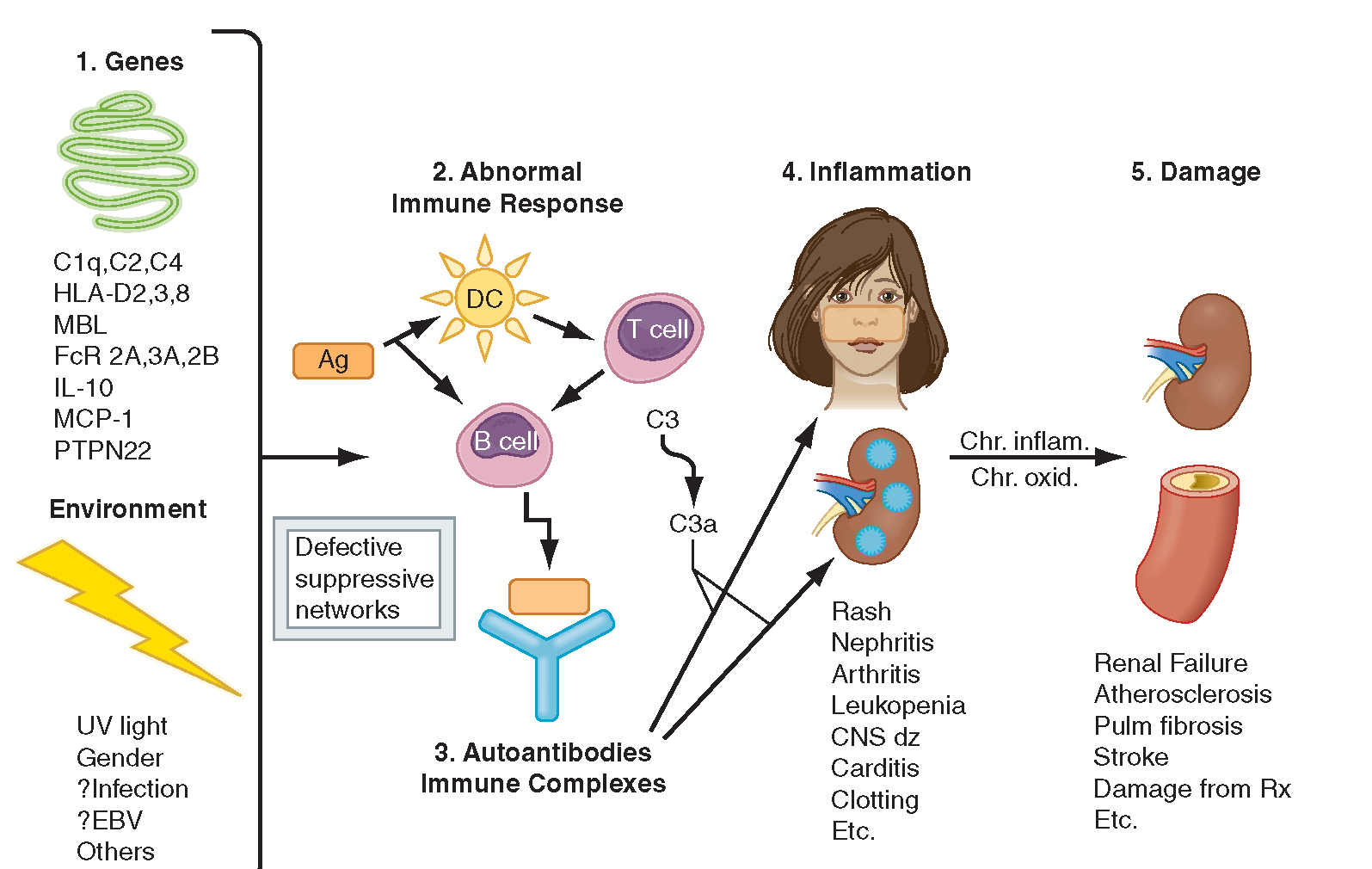
As a rare disease, Dr. House and his fictional diagnostics team are generally correct when they say ‘It’s never Lupus‘. However, for 20-70 individuals per 100,000 across the globe, sometimes the culprit really is the chronic and debilitating systemic lupus erythmatosus (SLE).
SLE is a complex chronic disease caused by the auto-immune inflammation of tissues, with an unpredictable sequence of effects on multiple organs. Standard treatment for SLE includes immuno-suppressants (cyclophosphamides and coritcosteroids) and palliative care for periods of crisis (termed ‘flares’). A more in-depth blog from the Wellcome Trust summarizes SLE here.
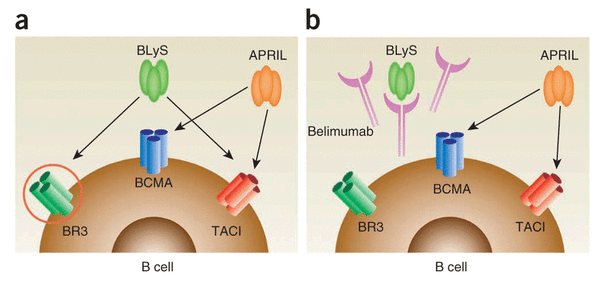
Lupus is a poorly tackled disease, and the first therapy to reach the market in 50 years was only in 2011: when GSK acquired marketing rights to Belimumab. This was a monoclonal antibody developed to treat SLE by Cambridge Antibody Technology (from the UK – later acquired by MedImmune) in collaboration with Human Genome Sciences (HGS). HGS was also bought out by GSK in 2012 for $3.6Bn. Evidently, the hunt for SLE treatments is crazy lucrative!
However, the problem with Belimumab was that it wasn’t so effective in trials for SLE patients with more severe organ damage (i.e. kidney and CNS), and it showed a low efficacy for its phase II trials to treat autoimmune rheumatoid arthritis. This further rooted the US FDA’s concerns over its general efficacy in Autoimmune disease…not to mention the high cost for each treatment (estimated at around £61,000 per year in the UK)!
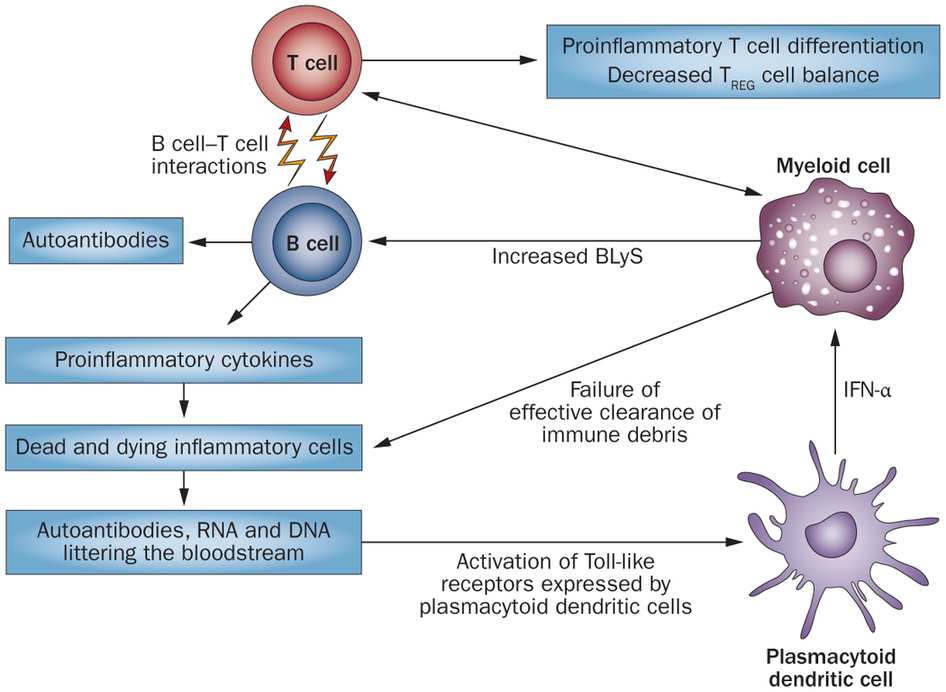
Thus, the search for a new immunotherapy resumed. The Lupus Research Institute‘s work by Kenneth Smith’s lab at the University of Cambridge found a new biomarker for relapses in SLE (as well as other autoimmune diseases such as Crohn’s and Ulcerative colitis), the findings of which were published in Nature.
Thanks to such research, new targets for autoimmune immunotherapies have been elucidated and honed in on by biotechs like arGEN-X. This is particularly encouraging, given recent therapeutic development failures in the biopharma world, which has also struggled to tackle this rare disease…Belgian UCB’s phase III candidate Epratuzumab being the latest tragedy (which had fast-track approval from the FDA!).
ArGEN-X’s latest candidate has now been successfully administered to the first human patients in its phase I trial.
ARGX-113 is the Fc-portion of an antibody that has been modified by arGEN-X’s proprietary ABDEG technology (akin to Ablynx’s Alpaca antibody platform), which increases its target affinity beyond that of normal IgG antibodies. As a result, ARGX-113 blocks antibody recycling and leads to faster clearance of IgG auto-antibodies (and therefore reducing inflammation).
The Phase 1 study is a double-blind and placebo-controlled study in healthy volunteers, which aims to achieve a safety and tolerability profile for the ARGX-113, with results expected in H1 of 2016.
So in the fight-against-the-body’s-own-fight-against-itself (phew!), we anticipate arGEN-X’s progress with bated breath..
UPDATE 28/11/15:
ArgenX’s phase I trial for Argx-113 remains Unspecified for a particular indication – with a general aim of reducing inflammation in Autoimmune crises for a range of chronic diseases; including SLE and Myasthenia Gravis.
However, recent presentations on the 17th November suggest ArgenX could now be leaning more towards MG as a specific indication for Argx-113, although this has yet to be confirmed. Watch this space.
‘Dr. Gregory House’ Featured Artwork courtesy of Adam Leonhardt.
Are you interested in antibody therapy R&D?







