Newsletter Signup - Under Article / In Page
"*" indicates required fields
Based in Cologne, Germany, Cevec focuses on human cell-based expression system for production of biological therapies. We’ve already covered their breakthroughs for safer gene therapy and the potential of CAP-GT for expression of viral vectors.
Besides CAP-GT, CEVEC has also developed a cell line that can produce complex glycosylated proteins. A good example of glycosylated protein is the therapeutic plasma protein. So far, these proteins have been isolated from donated human plasma by companies like Octapharma (a big company in Zurich’s Biotech ecosystem). With Cevec’s CAP-Go, recombinant plasma proteins can be produced for the first time.
I asked Nicole Faust (Cevec’s CSO) about the potential and application of this system.
CAP-GO enables the production of proteins that were previously out of reach for recombinant technologies. What makes your cell lines so unique?
Our major goal when developing the CAP-Go cell lines was to generate a platform that could efficiently produce complex glycoproteins at high concentrations and quality.
At the time, the field was focusing on antibody production with Chinese hamster ovary (CHO) cells. But then production of complex glycoproteins became of interest and the limitations of rodent cell lines (like CHO) became apparent. A human cell line was necessary. A possibility was HEK293 (also used to produce viral vectors), but these cells are poorly documented. This is a big obstacle for getting therapeutic proteins approved by regulators.
CAP-Go overcomes these deficiencies. Besides being capable of producing fully glycosylated recombinant proteins, the cells are very well documented, grow in suspension (free of serum and animal components) and produce high titers of recombinant protein.

Plasma proteins are an example of CAP-Go potential application. Will the production of plasma proteins move entirely towards recombinant production in the future? How will this play out?
Plasma fractionation will certainly continue to play an important role for selected products and applications, like in plasma-derived immunoglobulin mixtures.
However, there appears to be a growing trend towards recombinant protein production. Cevec has been approached by several international plasma fractionators, looking for suitable platforms for recombinant production. One example is our collaboration with Biotest (Germany) for recombinant production of tailor-made glycoproteins in the field of hemophilia.
The switch to recombinant protein production of plasma proteins is partially driven by economic, scalability and safety reasons. But the major driver is the increasing interest to develop improved, modified versions of therapeutic plasma proteins with a prolonged half-life, higher efficacy or reduced immunogenicity.
How can CAP-Go deliver these improved versions of glycoproteins?
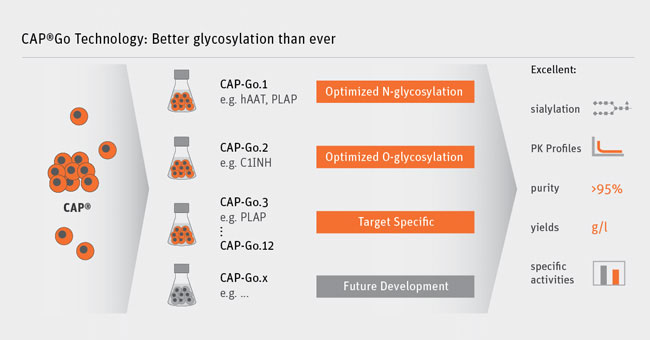
The first cell line we developed for our CAP Go expression platform is the CAP-Go.basic cell line which delivers a fully human glycosylation pattern displaying different glycan forms.
Since then, we have developed more than 10 CAP-Go cell lines to supply these tailored versions of proteins. Each cell line has been genetically modified to drive the glycosylation pattern towards a specific desired profile.
Rather than using random mutagenesis and screening, we generated these cell lines by introducing defined modifications in order to maintain a characterized genetic background of our cells.
One example is the CAP-Go.2 cell line that specifically addresses O-Glycans. These are very often neglected but are very important for the pharmacokinetic properties of several plasma proteins, including Factor VIII (needed for Hemophilia A) and C1 Inhibitor. In the latter case, we have been able to develop the first recombinant C1 Inhibitor with a half-life competitive to its plasma-derived counterpart.
CAP-Go also enables you to produce additional complex molecules, such as virus envelope proteins and proteins used for culturing stem cells. How could this impact the development of future therapies?
You are bringing up a very interesting point here. For example, one of the proteins that has been successfully produced on our CAP-Go platform is the HIV gp120 protein. On one hand, this protein can serve as a therapy by itself – it’s currently being developed to treat graft versus host disease.
On the other hand, the CAP-Go technology does not just produce the envelope proteins but also efficiently generates virus-like particles (VLPs). These are non-infectious viral particles, which do not possess any genetic information and therefore cannot replicate. They can be recognized by the immune system though and are thus a promising approach to vaccination.
With respect to the cell therapy product sector, we are producing laminins for BioLamina (Stockholm). CellGenix (Freiburg) utilizes our platform for the expression of Hepatocyte Growth Factor.
Both molecules are used for ex-vivo cell therapy applications and their production in other expression systems was so far notoriously difficult, if not impossible.
As we discussed previously, you have deals with CMOs to make CAP-GT available. We had the impression that Cevec intends to keep CAP-Go services more as an in-house service. Is that correct?
Our main ambition is to maximize the benefit of our technology for our clients. To this end, we have selected preferred CMO partners carefully. This is the case of our deal with Paragon Bioservices (US), which makes part of the both CAP-GT and CAP-Go roll-out in North America.
In the case of CAP-Go, Cevec has a unique expertise in cell line development. Accordingly, this initial part of a project is exclusively performed at our premises in Cologne, Germany.
About subsequent development, the decision to continue with Cevec or a CMO is agreed with the client. In particular, GMP manufacturing is the responsibility of our CMO partners. CEVEC helps ensure a smooth transition and technology transfer from one site to the next and capitalizes on its long-standing experience in optimizing the performance of our cells.
CAP-Go seems like a game-changer for production of recombinant proteins. We’ll certainly keep an eye on the development of CEVEC and its technology.
To learn more visit their website here.
Feature Image Credit: Nicole Faust (Source: Cevec)