Newsletter Signup - Under Article / In Page
"*" indicates required fields
We decided to make a visit to Espoo, Finland, this week, to visit Desentum. The company is developing allergy vaccines that could be safer and faster than approved vaccines.
Mission: To make a vaccine that decreases the time taken to reduce allergies compared with approved vaccines, which contain the natural allergen. Desentum plans to do this by injecting less dangerous forms of the allergen, such as birch pollen, helping the immune system to tone down its response to the natural allergen more quickly.
Allergies are a common health problem in developed countries, and there is a big need for effective, long-term treatments. “In Europe, they already affect 150 million people and this number is growing,” a spokesperson from Desentum told me.
“Currently, allergy is mostly managed by avoiding the allergen or taking allergy drugs, but avoidance is not always feasible and medication — such as antihistamines or corticosteroids — only offers temporary symptom relief.”
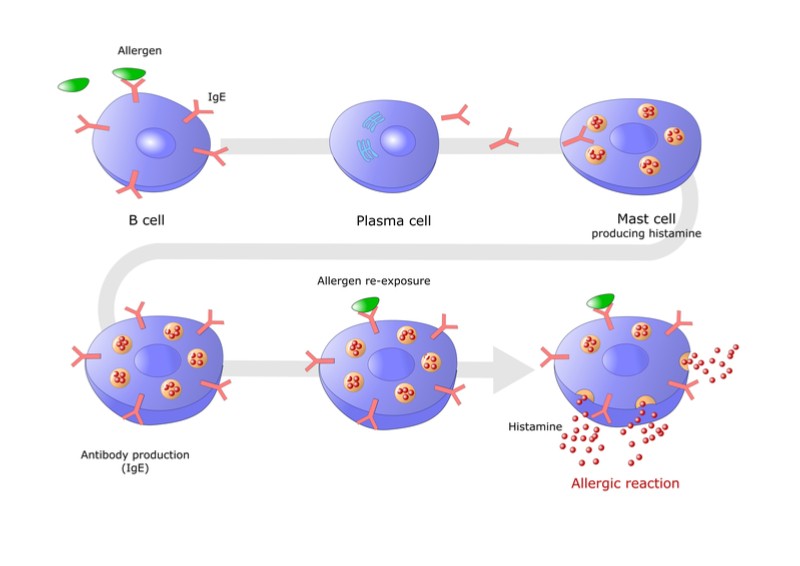
A form of long-term treatment for allergies is immunotherapy, or vaccination, where the allergen is injected at low doses, which are increased slowly to reduce the immune system’s allergic response. The reduction of allergy symptoms can take as long as a year, and can risk serious reactions such as anaphylactic shock. This is the reason why Desentum has designed its own vaccine platform to shorten the time of the therapy and make it safer.

With a focus on birch pollen, a common allergen in hay fever, Desentum’s approach is to produce weaker allergens, called hypoallergens, and use them in the vaccination process instead of the natural substance. While modifying allergens is a common technique in allergy vaccines, the spokesman told me that Desentum’s approach keeps the modifications as close to the natural structure as possible, which helps the hypoallergen to prime the immune system properly.
“Our allergens are designed to be less allergenic and therefore we anticipate that higher doses of them can be used, making the treatment safer, faster and more efficient,” Desentum’s spokesperson told me.
Desentum’s hypoallergens are made by genetically modified bacteria and designed to avoid triggering immunoglobulin E antibodies, a group of antibodies involved in allergic reactions. “In this process, the body starts producing specific protective immunoglobulin G antibodies that recognize the allergen and help eliminate it without triggering an allergic reaction,” continued the spokesperson.
The technology is going to be tested for the first time in humans with allergies to birch pollen in 2019, and Desentum hopes that it will enter the market by 2025, if all goes according to plan. In addition, the company has other applications in the works, including peanut, dog and horse allergies.
To fund this development, Desentum has raised €2.3M in a crowdfunding campaign in 2017, and another €1.9M from the EU’s Horizon 2020 programme.
What we think:
More effective allergy vaccines could improve the quality of life of millions of people worldwide, and could even save lives in many cases.
Notwithstanding, the world of allergy vaccine development is a difficult one. For one thing, there are lots of competitors. The UK biotech Allergy Therapeutics plans to enter phase III with its grass pollen hay fever vaccine next year. The German subsidiary of Allergy Therapeutics, Bencard Allergie, is running its own phase III trial for its birch pollen vaccine.
Another issue is there have been failures in the clinic for allergy vaccines. The UK company Circassia’s immunotherapy for cat allergies failed phase III in 2016. Furthermore, an immunotherapy treating peanut allergies failed in a phase III trial run by the French company DBV Technologies last year.
Nevertheless, Desentum is confident that its technology is competitive and likely to triumph in the clinic.
“Our technology is unique, and it has a strong scientific basis,” the spokesman said. “Ten years ago our research team made ground-breaking discoveries about a molecular complex that triggers an allergic reaction. We are utilizing that information to modify the allergens in a way no-one else has thought of before. Of course, the concept still needs to be tested in clinical trials but the preclinical results have been very promising.”
Images from Shutterstock






